[English] 日本語
 Yorodumi
Yorodumi- PDB-2kh9: Solution structure of yeast Prp24-RRM2 bound to a fragment of U6 RNA -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2kh9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Solution structure of yeast Prp24-RRM2 bound to a fragment of U6 RNA | ||||||
 Components Components |
| ||||||
 Keywords Keywords | SPLICING/RNA / Protein/RNA / RRM / snRNP / Splicing / mRNA processing / mRNA splicing / Nucleus / Phosphoprotein / RNA-binding / SPLICING-RNA COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationU6 snRNP / snRNA binding / spliceosomal complex assembly / spliceosomal tri-snRNP complex assembly / U6 snRNA binding / spliceosomal complex / mRNA splicing, via spliceosome / ribonucleoprotein complex / RNA binding / nucleus Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | SOLUTION NMR / simulated annealing, torsion angle dynamics | ||||||
| Model details | lowest energy, model 1 | ||||||
 Authors Authors | Martin-Tumasz, S.A. / Butcher, S.E. | ||||||
 Citation Citation |  Journal: Rna / Year: 2010 Journal: Rna / Year: 2010Title: Structure and functional implications of a complex containing a segment of U6 RNA bound by a domain of Prp24. Authors: Martin-Tumasz, S. / Reiter, N.J. / Brow, D.A. / Butcher, S.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2kh9.cif.gz 2kh9.cif.gz | 324.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2kh9.ent.gz pdb2kh9.ent.gz | 264.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2kh9.json.gz 2kh9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kh/2kh9 https://data.pdbj.org/pub/pdb/validation_reports/kh/2kh9 ftp://data.pdbj.org/pub/pdb/validation_reports/kh/2kh9 ftp://data.pdbj.org/pub/pdb/validation_reports/kh/2kh9 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 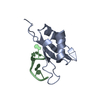
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 10841.389 Da / Num. of mol.: 1 / Fragment: UNP residues 115-197 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: PRP24, YMR268C, YM8156.10C / Plasmid: pET21b / Production host:  |
|---|---|
| #2: RNA chain | Mass: 1939.237 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: Synthetic RNA |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sample conditions | Ionic strength: 50 / pH: 7 / Pressure: ambient / Temperature: 298.15 K |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: simulated annealing, torsion angle dynamics / Software ordinal: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| NMR constraints | NOE constraints total: 1466 / NOE intraresidue total count: 230 / NOE long range total count: 427 / NOE medium range total count: 353 / NOE sequential total count: 456 / Hydrogen bond constraints total count: 43 / Protein chi angle constraints total count: 0 / Protein other angle constraints total count: 0 / Protein phi angle constraints total count: 43 / Protein psi angle constraints total count: 43 | ||||||||||||||||||||||||||||||||||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||||||||||||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 100 / Conformers submitted total number: 10 / Maximum lower distance constraint violation: 0 Å / Maximum torsion angle constraint violation: 7.5 ° / Maximum upper distance constraint violation: 0.38 Å / Torsion angle constraint violation method: HADDOCK Output | ||||||||||||||||||||||||||||||||||||||||||||||||
| NMR ensemble rms | Distance rms dev: 0.015 Å / Distance rms dev error: 0.002 Å |
 Movie
Movie Controller
Controller












 PDBj
PDBj HSQC
HSQC