Entry Database : PDB / ID : 2k20Title Solution structure of Par-3 PDZ3 in complex with PTEN peptide Partitioning-defective 3 homolog Protein tyrosine phosphatase and tensin homolog Keywords / / / / / / / / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Rattus norvegicus (Norway rat)Method / Model type details minimized average Authors Feng, W. / Wu, H. / Chan, L. / Zhang, M. Journal : To be Published Title : Solution structure of Par-3 PDZ3 in complex with PTEN peptideAuthors : Feng, W. / Wu, H. / Chan, L. / Zhang, M. History Deposition Mar 18, 2008 Deposition site / Processing site Revision 1.0 Jun 10, 2008 Provider / Type Revision 1.1 Jul 13, 2011 Group Revision 1.2 Mar 16, 2022 Group / Database references / Derived calculationsCategory database_2 / pdbx_nmr_software ... database_2 / pdbx_nmr_software / pdbx_struct_assembly / pdbx_struct_oper_list Item / _database_2.pdbx_database_accession / _pdbx_nmr_software.nameRevision 1.3 May 29, 2024 Group / Category / chem_comp_bond
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 Authors
Authors Citation
Citation Journal: To be Published
Journal: To be Published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2k20.cif.gz
2k20.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2k20.ent.gz
pdb2k20.ent.gz PDB format
PDB format 2k20.json.gz
2k20.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/k2/2k20
https://data.pdbj.org/pub/pdb/validation_reports/k2/2k20 ftp://data.pdbj.org/pub/pdb/validation_reports/k2/2k20
ftp://data.pdbj.org/pub/pdb/validation_reports/k2/2k20 Links
Links Assembly
Assembly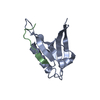
 Components
Components



 Sample preparation
Sample preparation Processing
Processing Movie
Movie Controller
Controller














 PDBj
PDBj





 NMRPipe
NMRPipe