+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2jre | ||||||
|---|---|---|---|---|---|---|---|
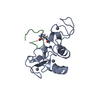
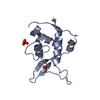
| Title | C60-1, a PDZ domain designed using statistical coupling analysis | ||||||
 Components Components | C60-1 PDZ domain peptide | ||||||
 Keywords Keywords | DE NOVO PROTEIN / C60-1 / PDZ | ||||||
| Function / homology | PDZ domain / Pdz3 Domain / Roll / Mainly Beta Function and homology information Function and homology information | ||||||
| Method | SOLUTION NMR / simulated annealing | ||||||
 Authors Authors | Larson, C. / Stiffler, M. / Li, P. / Rosen, M. / MacBeath, G. / Ranganathan, R. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: C60-1, a PDZ domain designed using statistical coupling analysis Authors: Larson, C. / Stiffler, M. / Li, P. / Rosen, M. / MacBeath, G. / Ranganathan, R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2jre.cif.gz 2jre.cif.gz | 872.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2jre.ent.gz pdb2jre.ent.gz | 744 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2jre.json.gz 2jre.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jr/2jre https://data.pdbj.org/pub/pdb/validation_reports/jr/2jre ftp://data.pdbj.org/pub/pdb/validation_reports/jr/2jre ftp://data.pdbj.org/pub/pdb/validation_reports/jr/2jre | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein | Mass: 11589.026 Da / Num. of mol.: 1 / Source method: obtained synthetically Details: The peptide is a synthetic construct designed using statistical coupling analysis and expressed in Escherichia coli BL21 cells using the pHis8.3 vector. |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sample conditions | pH: 6.5 / Temperature: 288 K |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: simulated annealing / Software ordinal: 1 | ||||||||||||||||||||||||
| NMR constraints | NOE constraints total: 1718 / NOE intraresidue total count: 675 / NOE long range total count: 430 / NOE medium range total count: 152 / NOE sequential total count: 378 / Hydrogen bond constraints total count: 64 / Protein phi angle constraints total count: 51 / Protein psi angle constraints total count: 51 | ||||||||||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 50 / Conformers submitted total number: 25 |
 Movie
Movie Controller
Controller










 PDBj
PDBj
 HSQC
HSQC