+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2i9m | ||||||
|---|---|---|---|---|---|---|---|
| Title | Design of a-helix based on conformationally restricted libraries | ||||||
 Components Components | MHA6 | ||||||
 Keywords Keywords | DE NOVO PROTEIN / HELIX | ||||||
| Method | SOLUTION NMR / torsion angle dynamics | ||||||
 Authors Authors | Pantoja-Uceda, D. / Pineda-Lucena, A. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Design of minimal independent protein motifs able to fold autonomously Authors: Pantoja-Uceda, D. / Pastor, M.T. / Salgado, J. / Pineda-Lucena, A. / Perez-Paya, E. | ||||||
| History |
|
- Structure visualization
Structure visualization
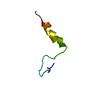
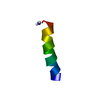
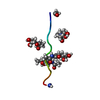
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2i9m.cif.gz 2i9m.cif.gz | 104 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2i9m.ent.gz pdb2i9m.ent.gz | 78.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2i9m.json.gz 2i9m.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2i9m_validation.pdf.gz 2i9m_validation.pdf.gz | 334.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2i9m_full_validation.pdf.gz 2i9m_full_validation.pdf.gz | 433.3 KB | Display | |
| Data in XML |  2i9m_validation.xml.gz 2i9m_validation.xml.gz | 7.1 KB | Display | |
| Data in CIF |  2i9m_validation.cif.gz 2i9m_validation.cif.gz | 10.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i9/2i9m https://data.pdbj.org/pub/pdb/validation_reports/i9/2i9m ftp://data.pdbj.org/pub/pdb/validation_reports/i9/2i9m ftp://data.pdbj.org/pub/pdb/validation_reports/i9/2i9m | HTTPS FTP |
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 1740.998 Da / Num. of mol.: 1 / Source method: obtained synthetically Details: the designed peptide was synthesized using Fmoc chemistry |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||||||
| NMR details | Text: This structure was determined using standard 2D homonuclear techniques |
- Sample preparation
Sample preparation
| Details |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample conditions | pH: 5 / Pressure: ambient / Temperature: 283 K |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| NMR spectrometer | Type: Bruker AVANCE / Manufacturer: Bruker / Model: AVANCE / Field strength: 600 MHz |
- Processing
Processing
| NMR software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: torsion angle dynamics / Software ordinal: 1 Details: the structures are based on a total of 171 NOE-derived distance constraints. | ||||||||||||||||
| NMR representative | Selection criteria: lowest target function | ||||||||||||||||
| NMR ensemble | Conformer selection criteria: target function / Conformers calculated total number: 100 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller










 PDBj
PDBj
 CYANA
CYANA