[English] 日本語
 Yorodumi
Yorodumi- PDB-2hb9: Crystal Structure of the Zinc-Beta-Lactamase L1 from Stenotrophom... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2hb9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the Zinc-Beta-Lactamase L1 from Stenotrophomonas Maltophilia (Inhibitor 3) | ||||||
 Components Components | Metallo-beta-lactamase L1 | ||||||
 Keywords Keywords | HYDROLASE / METALLO / ZN / LACTAMASE | ||||||
| Function / homology |  Function and homology information Function and homology informationantibiotic catabolic process / beta-lactamase activity / beta-lactamase / periplasmic space / response to antibiotic / zinc ion binding Similarity search - Function | ||||||
| Biological species |  Stenotrophomonas maltophilia (bacteria) Stenotrophomonas maltophilia (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.75 Å MOLECULAR REPLACEMENT / Resolution: 1.75 Å | ||||||
 Authors Authors | Nauton, L. / Garau, G. / Kahn, R. / Dideberg, O. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2008 Journal: J.Mol.Biol. / Year: 2008Title: Structural insights into the design of inhibitors for the L1 metallo-beta-lactamase from Stenotrophomonas maltophilia. Authors: Nauton, L. / Kahn, R. / Garau, G. / Hernandez, J.F. / Dideberg, O. #1:  Journal: Antimicrob.Agents Chemother. / Year: 2004 Journal: Antimicrob.Agents Chemother. / Year: 2004Title: Update of the Standard Numbering Scheme for Class B Beta-Lactamases Authors: Garau, G. / Garcia-Saez, I. / Bebrone, C. / Anne, C. / Mercuri, P. / Galleni, M. / Frere, J.-M. / Dideberg, O. #2:  Journal: Embo J. / Year: 1995 Journal: Embo J. / Year: 1995Title: The 3-D Structure of a Zinc Metallo-Beta-Lactamase from Bacillus Cereus Reveals a New Type of Protein Fold Authors: Carfi, A. / Pares, S. / Duee, E. / Galleni, M. / Duez, C. / Frere, J.-M. / Dideberg, O. | ||||||
| History |
| ||||||
| Remark 999 | SEQUENCE THE RESIDUE NUMBERING OF THE COORDINATES IS NON-SEQUENTIAL. MANY NUMBERS WERE SIMPLY ... SEQUENCE THE RESIDUE NUMBERING OF THE COORDINATES IS NON-SEQUENTIAL. MANY NUMBERS WERE SIMPLY SKIPPED IN THE NUMBERING AND HAVE NOTHING TO DO WITH LACK OF ELECTRON DENSITY. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2hb9.cif.gz 2hb9.cif.gz | 71.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2hb9.ent.gz pdb2hb9.ent.gz | 51.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2hb9.json.gz 2hb9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2hb9_validation.pdf.gz 2hb9_validation.pdf.gz | 455.7 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2hb9_full_validation.pdf.gz 2hb9_full_validation.pdf.gz | 457.3 KB | Display | |
| Data in XML |  2hb9_validation.xml.gz 2hb9_validation.xml.gz | 14 KB | Display | |
| Data in CIF |  2hb9_validation.cif.gz 2hb9_validation.cif.gz | 20.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hb/2hb9 https://data.pdbj.org/pub/pdb/validation_reports/hb/2hb9 ftp://data.pdbj.org/pub/pdb/validation_reports/hb/2hb9 ftp://data.pdbj.org/pub/pdb/validation_reports/hb/2hb9 | HTTPS FTP |
-Related structure data
| Related structure data |  2fm6C  2fu7C  2fu8C  2fu9C  2gfjC  2gfkC  2h6aC  1smlS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 28740.453 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Stenotrophomonas maltophilia (bacteria) Stenotrophomonas maltophilia (bacteria)Strain: IID 1275 / Cellular location: PERIPLASM / Gene: L1 / Plasmid: PET26 / Production host:  | ||||||||
|---|---|---|---|---|---|---|---|---|---|
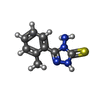
| #2: Chemical | | #3: Chemical | #4: Chemical | #5: Water | ChemComp-HOH / | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 51 % |
|---|---|
| Crystal grow | Temperature: 281 K / Method: vapor diffusion, hanging drop / pH: 7.75 Details: 1.8M AMMONIUM SULFATE, 0.1M HEPES PH 7.75, 1.5% V/V PEG 400, VAPOR DIFFUSION, HANGING DROP, temperature 281K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: ENRAF-NONIUS FR571 / Wavelength: 1.5418 / Wavelength: 1.5418 Å ROTATING ANODE / Type: ENRAF-NONIUS FR571 / Wavelength: 1.5418 / Wavelength: 1.5418 Å |
| Detector | Type: MAR scanner 345 mm plate / Detector: IMAGE PLATE / Date: Mar 15, 2006 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.75→19.51 Å / Num. all: 31993 / Num. obs: 31993 / % possible obs: 99.6 % / Observed criterion σ(I): 2 / Redundancy: 6.7 % / Rmerge(I) obs: 0.059 / Rsym value: 0.054 / Net I/σ(I): 11.2 |
| Reflection shell | Resolution: 1.75→1.84 Å / Redundancy: 5.7 % / Rmerge(I) obs: 0.381 / Mean I/σ(I) obs: 2 / Rsym value: 0.287 / % possible all: 99.6 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1SML Resolution: 1.75→19.67 Å / Cor.coef. Fo:Fc: 0.957 / Cor.coef. Fo:Fc free: 0.948 / SU B: 1.747 / SU ML: 0.057 / Cross valid method: THROUGHOUT / ESU R: 0.099 / ESU R Free: 0.093 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 16.876 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.75→19.67 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.75→1.795 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj