[English] 日本語
 Yorodumi
Yorodumi- PDB-2h6g: W102T Protein Farnesyltransferase Mutant Complexed with a Geranyl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2h6g | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | W102T Protein Farnesyltransferase Mutant Complexed with a Geranylgeranylated DDPTASACVLS Peptide Product at 1.85A Resolution | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | TRANSFERASE / FTase / farnesyltransferase / farnesyl transferase / prenyltransferase / CaaX / Ras / lipid modification / prenylation / substrate selectivity | |||||||||
| Function / homology |  Function and homology information Function and homology informationskeletal muscle acetylcholine-gated channel clustering / protein geranylgeranyltransferase activity / peptide pheromone maturation / protein farnesylation / protein geranylgeranyltransferase type I / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein farnesyltransferase / protein farnesyltransferase activity / protein farnesyltransferase complex ...skeletal muscle acetylcholine-gated channel clustering / protein geranylgeranyltransferase activity / peptide pheromone maturation / protein farnesylation / protein geranylgeranyltransferase type I / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein farnesyltransferase / protein farnesyltransferase activity / protein farnesyltransferase complex / Rab geranylgeranyltransferase activity / protein geranylgeranylation / positive regulation of skeletal muscle acetylcholine-gated channel clustering / acetyltransferase activator activity / microtubule associated complex / Apoptotic cleavage of cellular proteins / regulation of microtubule-based movement / positive regulation of Rac protein signal transduction / alpha-tubulin binding / positive regulation of cell cycle / peptide binding / transforming growth factor beta receptor signaling pathway / lipid metabolic process / receptor tyrosine kinase binding / RAS processing / Inactivation, recovery and regulation of the phototransduction cascade / microtubule binding / Potential therapeutics for SARS / molecular adaptor activity / positive regulation of cell population proliferation / enzyme binding / zinc ion binding / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.85 Å MOLECULAR REPLACEMENT / Resolution: 1.85 Å | |||||||||
 Authors Authors | Terry, K.L. / Beese, L.S. | |||||||||
 Citation Citation |  Journal: Biochemistry / Year: 2006 Journal: Biochemistry / Year: 2006Title: Conversion of protein farnesyltransferase to a geranylgeranyltransferase. Authors: Terry, K.L. / Casey, P.J. / Beese, L.S. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2h6g.cif.gz 2h6g.cif.gz | 178.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2h6g.ent.gz pdb2h6g.ent.gz | 135.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2h6g.json.gz 2h6g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h6/2h6g https://data.pdbj.org/pub/pdb/validation_reports/h6/2h6g ftp://data.pdbj.org/pub/pdb/validation_reports/h6/2h6g ftp://data.pdbj.org/pub/pdb/validation_reports/h6/2h6g | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2h6fC  2h6hC  2h6iC  1jcqS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 44864.840 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: Human FTase alpha subunit / Species (production host): Escherichia coli / Production host: Homo sapiens (human) / Gene: Human FTase alpha subunit / Species (production host): Escherichia coli / Production host:  References: UniProt: P49354, protein farnesyltransferase, protein geranylgeranyltransferase type I |
|---|---|
| #2: Protein | Mass: 48737.301 Da / Num. of mol.: 1 / Mutation: W102T Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: Human FTase beta subunit / Species (production host): Escherichia coli / Production host: Homo sapiens (human) / Gene: Human FTase beta subunit / Species (production host): Escherichia coli / Production host:  |
-Protein/peptide / Sugars , 2 types, 2 molecules P
| #3: Protein/peptide | Mass: 1078.152 Da / Num. of mol.: 1 Fragment: residues 173-180 of Rap2a, residues187-189 of H-Ras Mutation: C176T, C177A / Source method: obtained synthetically / Details: Chemically synthesized |
|---|---|
| #4: Polysaccharide | beta-D-fructofuranose-(2-1)-alpha-D-glucopyranose / sucrose |
-Non-polymers , 3 types, 636 molecules 



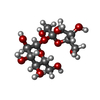
| #5: Chemical | ChemComp-ZN / |
|---|---|
| #6: Chemical | ChemComp-GER / |
| #7: Water | ChemComp-HOH / |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.34 Å3/Da / Density % sol: 63.2 % |
|---|---|
| Crystal grow | Temperature: 290 K / Method: vapor diffusion, hanging drop / pH: 5.2 Details: 14% PEG 8000, 200 mM ammonium acetate pH 5.2, 20 mM DTT, VAPOR DIFFUSION, HANGING DROP, temperature 290K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE / Date: Apr 10, 2005 / Details: double mirror optics (MSC) |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.85→50 Å / Num. all: 103157 / Num. obs: 98678 / % possible obs: 98.3 % / Observed criterion σ(I): -3 / Redundancy: 3.8 % / Biso Wilson estimate: 21.7 Å2 / Rsym value: 0.071 / Net I/σ(I): 16.2 |
| Reflection shell | Resolution: 1.85→1.92 Å / Redundancy: 2.3 % / Mean I/σ(I) obs: 1.9 / Num. unique all: 9978 / Rsym value: 0.484 / % possible all: 97 |
- Processing
Processing
| Software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1JCQ Resolution: 1.85→42.88 Å / Isotropic thermal model: restrained / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||
| Displacement parameters | Biso mean: 23.8 Å2
| ||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.85→42.88 Å
| ||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||
| LS refinement shell | Resolution: 1.85→1.97 Å / Rfactor Rfree error: 0.01
|
 Movie
Movie Controller
Controller



















 PDBj
PDBj