[English] 日本語
 Yorodumi
Yorodumi- PDB-2agd: Crystal Structure of Human M340H-Beta-1,4-Galactosyltransferase-I... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2agd | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Human M340H-Beta-1,4-Galactosyltransferase-I(M340H-B4Gal-T1) in Complex with GlcNAc-beta1,4-Man-alpha1,3-Man-beta-OR | |||||||||
 Components Components | Beta-1,4-galactosyltransferase 1 | |||||||||
 Keywords Keywords | TRANSFERASE / beta1 / 4-Galactosyltransferase-I / Trisaccharide / closed conformation / Mutant | |||||||||
| Function / homology |  Function and homology information Function and homology informationDefective B4GALT1 causes CDG-2d / galactosyltransferase activity / Interaction With Cumulus Cells And The Zona Pellucida / Defective B4GALT1 causes B4GALT1-CDG (CDG-2d) / Lactose synthesis / Keratan sulfate biosynthesis / lactose synthase / neolactotriaosylceramide beta-1,4-galactosyltransferase / beta-N-acetylglucosaminylglycopeptide beta-1,4-galactosyltransferase / N-acetyllactosamine synthase ...Defective B4GALT1 causes CDG-2d / galactosyltransferase activity / Interaction With Cumulus Cells And The Zona Pellucida / Defective B4GALT1 causes B4GALT1-CDG (CDG-2d) / Lactose synthesis / Keratan sulfate biosynthesis / lactose synthase / neolactotriaosylceramide beta-1,4-galactosyltransferase / beta-N-acetylglucosaminylglycopeptide beta-1,4-galactosyltransferase / N-acetyllactosamine synthase / N-acetyllactosamine synthase activity / positive regulation of circulating fibrinogen levels / beta-N-acetylglucosaminylglycopeptide beta-1,4-galactosyltransferase activity / Golgi trans cisterna / N-Glycan antennae elongation / penetration of zona pellucida / UDP-galactosyltransferase activity / regulation of acrosome reaction / lactose synthase activity / lactose biosynthetic process / macrophage migration / oligosaccharide biosynthetic process / development of secondary sexual characteristics / desmosome / acute inflammatory response / galactose metabolic process / Pre-NOTCH Processing in Golgi / positive regulation of epithelial cell proliferation involved in wound healing / binding of sperm to zona pellucida / protein N-linked glycosylation / angiogenesis involved in wound healing / Transferases; Glycosyltransferases; Hexosyltransferases / Golgi cisterna membrane / azurophil granule membrane / epithelial cell development / alpha-tubulin binding / beta-tubulin binding / extracellular matrix organization / secretory granule membrane / epithelial cell proliferation / filopodium / lipid metabolic process / brush border membrane / negative regulation of epithelial cell proliferation / manganese ion binding / basolateral plasma membrane / cell adhesion / positive regulation of apoptotic process / Golgi membrane / external side of plasma membrane / Neutrophil degranulation / Golgi apparatus / protein-containing complex / extracellular space / extracellular exosome / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | |||||||||
 Authors Authors | Ramasamy, V. / Ramakrishnan, B. / Boeggeman, E. / Ratner, D.M. / Seeberger, P.H. / Qasba, P.K. | |||||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2005 Journal: J.Mol.Biol. / Year: 2005Title: Oligosaccharide Preferences of beta1,4-Galactosyltransferase-I: Crystal Structures of Met340His Mutant of Human beta1,4-Galactosyltransferase-I with a Pentasaccharide and Trisaccharides of the N-Glycan Moiety Authors: Ramasamy, V. / Ramakrishnan, B. / Boeggeman, E. / Ratner, D.M. / Seeberger, P.H. / Qasba, P.K. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2agd.cif.gz 2agd.cif.gz | 199.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2agd.ent.gz pdb2agd.ent.gz | 157.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2agd.json.gz 2agd.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ag/2agd https://data.pdbj.org/pub/pdb/validation_reports/ag/2agd ftp://data.pdbj.org/pub/pdb/validation_reports/ag/2agd ftp://data.pdbj.org/pub/pdb/validation_reports/ag/2agd | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2ae7C  2aecC  2aesC  2ah9C  1o0rS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||
| 2 | 
| ||||||||||
| 3 | 
| ||||||||||
| Unit cell |
|
- Components
Components
-Protein / Sugars , 2 types, 6 molecules ABC
| #1: Protein | Mass: 32773.230 Da / Num. of mol.: 3 / Fragment: Catalytic domain, Residues 126-398 / Mutation: M340H, C338T, R337T Source method: isolated from a genetically manipulated source Details: N-acetyllactosamine synthase part / Source: (gene. exp.)  Homo sapiens (human) Homo sapiens (human)Description: N-terminal carries t7 tag of 11 amino acids followed by GLY, SER & ALA Gene: B4GALT1, GGTB2 / Plasmid: PET23A / Species (production host): Escherichia coli / Production host:  #2: Polysaccharide | Source method: isolated from a genetically manipulated source |
|---|
-Non-polymers , 7 types, 564 molecules 



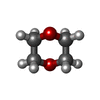








| #3: Chemical | | #4: Chemical | ChemComp-SO4 / #5: Chemical | #6: Chemical | ChemComp-GOL / #7: Chemical | ChemComp-DIO / | #8: Chemical | ChemComp-MES / | #9: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.8 Å3/Da / Density % sol: 67.3 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: mes, ammonium sulfate, dioxane, pH 6.5, VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X9B / Wavelength: 0.97946 Å / Beamline: X9B / Wavelength: 0.97946 Å |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Apr 23, 2004 / Details: Mirrors |
| Radiation | Monochromator: GRAPHITE / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97946 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→40 Å / Num. obs: 118434 / % possible obs: 100 % / Observed criterion σ(I): 1 / Redundancy: 6.1 % / Biso Wilson estimate: 17 Å2 / Rsym value: 0.063 / Net I/σ(I): 28.4 |
| Reflection shell | Resolution: 1.9→1.97 Å / Redundancy: 5.5 % / Mean I/σ(I) obs: 3.4 / Num. unique all: 11733 / Rsym value: 0.468 / % possible all: 99.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1O0R Resolution: 1.9→40 Å / Rfactor Rfree error: 0.002 / Data cutoff high absF: 3307113.13 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 45.3818 Å2 / ksol: 0.39085 e/Å3 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 28.9 Å2
| ||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→40 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.9→2.02 Å / Rfactor Rfree error: 0.006 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj









