[English] 日本語
 Yorodumi
Yorodumi- PDB-1tdr: EXPRESSION, CHARACTERIZATION, AND CRYSTALLOGRAPHIC ANALYSIS OF TE... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1tdr | ||||||
|---|---|---|---|---|---|---|---|
| Title | EXPRESSION, CHARACTERIZATION, AND CRYSTALLOGRAPHIC ANALYSIS OF TELLUROMETHIONYL DIHYDROFOLATE REDUCTASE | ||||||
 Components Components | TELLUROMETHIONYL DIHYDROFOLATE REDUCTASE | ||||||
 Keywords Keywords | OXIDOREDUCTASE | ||||||
| Function / homology |  Function and homology information Function and homology informationglycine biosynthetic process / methotrexate binding / dihydrofolic acid binding / 10-formyltetrahydrofolate biosynthetic process / response to methotrexate / folic acid biosynthetic process / folic acid binding / NADP+ binding / dihydrofolate metabolic process / dihydrofolate reductase ...glycine biosynthetic process / methotrexate binding / dihydrofolic acid binding / 10-formyltetrahydrofolate biosynthetic process / response to methotrexate / folic acid biosynthetic process / folic acid binding / NADP+ binding / dihydrofolate metabolic process / dihydrofolate reductase / dihydrofolate reductase activity / folic acid metabolic process / tetrahydrofolate biosynthetic process / NADPH binding / one-carbon metabolic process / NADP binding / response to xenobiotic stimulus / response to antibiotic / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 2.5 Å SYNCHROTRON / Resolution: 2.5 Å | ||||||
 Authors Authors | Lewinski, K. / Lebioda, L. | ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 1995 Journal: Acta Crystallogr.,Sect.D / Year: 1995Title: Expression, characterization and crystallographic analysis of telluromethionyl dihydrofolate reductase. Authors: Boles, J.O. / Lewinski, K. / Kunckle, M.G. / Hatada, M. / Lebioda, L. / Dunlap, R.B. / Odom, J.D. #1:  Journal: Nat.Struct.Biol. / Year: 1994 Journal: Nat.Struct.Biol. / Year: 1994Title: Bio-Incorporation of Telluromethionine Into Buried Residues of Dihydrofolate Reductase Authors: Boles, J.O. / Lewinski, K. / Kunkle, M. / Odom, J.D. / Dunlap, R.B. / Lebioda, L. / Hatada, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1tdr.cif.gz 1tdr.cif.gz | 88 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1tdr.ent.gz pdb1tdr.ent.gz | 65.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1tdr.json.gz 1tdr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1tdr_validation.pdf.gz 1tdr_validation.pdf.gz | 505.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1tdr_full_validation.pdf.gz 1tdr_full_validation.pdf.gz | 552.8 KB | Display | |
| Data in XML |  1tdr_validation.xml.gz 1tdr_validation.xml.gz | 16.4 KB | Display | |
| Data in CIF |  1tdr_validation.cif.gz 1tdr_validation.cif.gz | 22.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/td/1tdr https://data.pdbj.org/pub/pdb/validation_reports/td/1tdr ftp://data.pdbj.org/pub/pdb/validation_reports/td/1tdr ftp://data.pdbj.org/pub/pdb/validation_reports/td/1tdr | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: GLY A 95 - GLY A 96 OMEGA = 359.20 PEPTIDE BOND DEVIATES SIGNIFICANTLY FROM TRANS CONFORMATION 2: GLY B 95 - GLY B 96 OMEGA = 359.75 PEPTIDE BOND DEVIATES SIGNIFICANTLY FROM TRANS CONFORMATION |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 18020.393 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  References: UniProt: P00379, UniProt: P0ABQ4*PLUS, dihydrofolate reductase |
|---|
-Non-polymers , 5 types, 349 molecules 
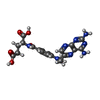







| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-TE / #5: Chemical | ChemComp-CA / | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.58 Å3/Da / Density % sol: 52.27 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 7.5 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X8C / Wavelength: 0.98 Å / Beamline: X8C / Wavelength: 0.98 Å |
|---|---|
| Detector | Detector: CCD / Date: Oct 24, 1992 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.98 Å / Relative weight: 1 |
| Reflection | Num. obs: 11234 / % possible obs: 88 % / Redundancy: 4.35 % / Rmerge(I) obs: 0.068 |
| Reflection | *PLUS Highest resolution: 2.5 Å / Num. measured all: 48880 / Rmerge(I) obs: 0.065 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.5→16 Å / σ(F): 0 Details: ATOM SD OF MET 42 AND ATOM SD OF MET 92 HAVE BEEN PARTIALLY CHEMICALLY SUBSTITUTED BY TELLURIUM. COORDINATES ARE GIVEN FOR BOTH SD AND TE IN THIS ENTRY, WITH PARTIAL OCCUPANCIES. NOTE THAT ...Details: ATOM SD OF MET 42 AND ATOM SD OF MET 92 HAVE BEEN PARTIALLY CHEMICALLY SUBSTITUTED BY TELLURIUM. COORDINATES ARE GIVEN FOR BOTH SD AND TE IN THIS ENTRY, WITH PARTIAL OCCUPANCIES. NOTE THAT IT IS NOT POSSIBLE FOR BOTH SD AND TE TO BE PRESENT SIMULTANEOUSLY IN ONE RESIDUE. ATOMS SD MET 42 AND SD MET 92 ARE PRESENTED AS PART OF RESIDUES 42 AND 92, RESPECTIVELY. THE TELLURIUM ATOMS ARE PRESENTED AS HET GROUPS AT THE END OF THE CHAIN.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→16 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: PROLSQ / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor Rwork: 0.124 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller













 PDBj
PDBj




