[English] 日本語
 Yorodumi
Yorodumi- PDB-1s8f: Crystal structure of Rab9 complexed to GDP reveals a dimer with a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1s8f | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Rab9 complexed to GDP reveals a dimer with an active conformation of switch II | ||||||
 Components Components | Ras-related protein Rab-9A | ||||||
 Keywords Keywords | PROTEIN TRANSPORT / intracellular transport / vesicular trafficking / hemihedral twinning | ||||||
| Function / homology |  Function and homology information Function and homology informationRetrograde transport at the Trans-Golgi-Network / RAB GEFs exchange GTP for GDP on RABs / RHOBTB3 ATPase cycle / RAB geranylgeranylation / Rab protein signal transduction / retrograde transport, endosome to Golgi / positive regulation of exocytosis / phagocytic vesicle / receptor-mediated endocytosis / small monomeric GTPase ...Retrograde transport at the Trans-Golgi-Network / RAB GEFs exchange GTP for GDP on RABs / RHOBTB3 ATPase cycle / RAB geranylgeranylation / Rab protein signal transduction / retrograde transport, endosome to Golgi / positive regulation of exocytosis / phagocytic vesicle / receptor-mediated endocytosis / small monomeric GTPase / phagocytic vesicle membrane / GDP binding / melanosome / late endosome / protein transport / regulation of protein localization / G protein activity / lysosome / Golgi membrane / GTPase activity / endoplasmic reticulum membrane / GTP binding / identical protein binding / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.77 Å MOLECULAR REPLACEMENT / Resolution: 1.77 Å | ||||||
 Authors Authors | Wittmann, J.G. / Rudolph, M.G. | ||||||
 Citation Citation |  Journal: Febs Lett. / Year: 2004 Journal: Febs Lett. / Year: 2004Title: Crystal structure of Rab9 complexed to GDP reveals a dimer with an active conformation of switch II. Authors: Wittmann, J.G. / Rudolph, M.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1s8f.cif.gz 1s8f.cif.gz | 93 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1s8f.ent.gz pdb1s8f.ent.gz | 67.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1s8f.json.gz 1s8f.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/s8/1s8f https://data.pdbj.org/pub/pdb/validation_reports/s8/1s8f ftp://data.pdbj.org/pub/pdb/validation_reports/s8/1s8f ftp://data.pdbj.org/pub/pdb/validation_reports/s8/1s8f | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1ky3S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| 2 | 
| |||||||||
| 3 | 
| |||||||||
| 4 |
| |||||||||
| 5 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 20037.398 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Details: C-terminal truncation / Source: (gene. exp.)   |
|---|
-Non-polymers , 6 types, 241 molecules 

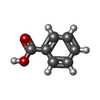








| #2: Chemical | | #3: Chemical | #4: Chemical | ChemComp-BEZ / #5: Chemical | ChemComp-MG / | #6: Chemical | ChemComp-CL / | #7: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.17 Å3/Da / Density % sol: 48.7 % |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: PEG8000, Na-benzoate, pH 6.5, VAPOR DIFFUSION, SITTING DROP, temperature 295K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: BW7A / Wavelength: 1.0091 Å / Beamline: BW7A / Wavelength: 1.0091 Å |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Jan 20, 2003 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.0091 Å / Relative weight: 1 |
| Reflection | Resolution: 1.77→30 Å / Num. obs: 37662 / % possible obs: 99.9 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.8 % / Biso Wilson estimate: 22.8 Å2 / Rsym value: 0.053 / Net I/σ(I): 24.8 |
| Reflection shell | Resolution: 1.77→1.83 Å / Redundancy: 3.8 % / Mean I/σ(I) obs: 2.5 / Num. unique all: 3752 / Rsym value: 0.0691 / % possible all: 99.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 1ky3 Resolution: 1.77→30 Å / Num. parameters: 12164 / Num. restraintsaints: 11650 / Isotropic thermal model: Isotropic / Cross valid method: FREE R / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber Details: The structure was refined against data from a hemihedrally twinned crystal. The twin fraction is 0.253, the twin operator is (k,h,-l).
| |||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 30 Å2 | |||||||||||||||||||||||||||||||||
| Refine analyze | Num. disordered residues: 4 / Occupancy sum hydrogen: 2550 / Occupancy sum non hydrogen: 3027.5
| |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.77→30 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj








