Entry Database : PDB / ID : 1r4iTitle Crystal Structure of Androgen Receptor DNA-Binding Domain Bound to a Direct Repeat Response Element 5'-D(*CP*CP*AP*GP*AP*AP*CP*AP*TP*CP*AP*AP*GP*AP*AP*CP*AP*G)-3'5'-D(*CP*TP*GP*TP*TP*CP*TP*TP*GP*AP*TP*GP*TP*TP*CP*TP*GP*G)-3'Androgen receptor Keywords / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Rattus norvegicus (Norway rat)Method / / / Resolution : 3.1 Å Authors Shaffer, P.L. / Jivan, A. / Dollins, D.E. / Claessens, F. / Gewirth, D.T. Journal : Proc.Natl.Acad.Sci.USA / Year : 2004Title : Structural basis of androgen receptor binding to selective androgen response elements.Authors : Shaffer, P.L. / Jivan, A. / Dollins, D.E. / Claessens, F. / Gewirth, D.T. History Deposition Oct 6, 2003 Deposition site / Processing site Revision 1.0 Jun 29, 2004 Provider / Type Revision 1.1 Apr 29, 2008 Group Revision 1.2 Jul 13, 2011 Group Revision 1.3 Oct 27, 2021 Group / Derived calculationsCategory database_2 / pdbx_struct_conn_angle ... database_2 / pdbx_struct_conn_angle / struct_conn / struct_ref_seq_dif / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id Revision 1.4 Aug 23, 2023 Group / Refinement descriptionCategory / chem_comp_bond / pdbx_initial_refinement_modelRevision 1.5 Oct 16, 2024 Group / Category / pdbx_modification_feature
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.1 Å
MOLECULAR REPLACEMENT / Resolution: 3.1 Å  Authors
Authors Citation
Citation Journal: Proc.Natl.Acad.Sci.USA / Year: 2004
Journal: Proc.Natl.Acad.Sci.USA / Year: 2004 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 1r4i.cif.gz
1r4i.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb1r4i.ent.gz
pdb1r4i.ent.gz PDB format
PDB format 1r4i.json.gz
1r4i.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/r4/1r4i
https://data.pdbj.org/pub/pdb/validation_reports/r4/1r4i ftp://data.pdbj.org/pub/pdb/validation_reports/r4/1r4i
ftp://data.pdbj.org/pub/pdb/validation_reports/r4/1r4i
 Links
Links Assembly
Assembly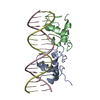
 Components
Components

 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 22-ID / Wavelength: 1.0000, 1.2831
/ Beamline: 22-ID / Wavelength: 1.0000, 1.2831 Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller









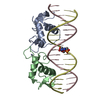


 PDBj
PDBj








































