[English] 日本語
 Yorodumi
Yorodumi- PDB-1pyo: Crystal Structure of Human Caspase-2 in Complex with Acetyl-Leu-A... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1pyo | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Human Caspase-2 in Complex with Acetyl-Leu-Asp-Glu-Ser-Asp-cho | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / APOPTOSIS / CASPASE / ALPHA-BETA / THIOL PROTEASE / HYDROLASE-HYDROLASE INHIBITOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationcaspase-2 / endopeptidase complex / NADE modulates death signalling / neural retina development / luteolysis / execution phase of apoptosis / TP53 Regulates Transcription of Caspase Activators and Caspases / ectopic germ cell programmed cell death / extrinsic apoptotic signaling pathway in absence of ligand / positive regulation of apoptotic signaling pathway ...caspase-2 / endopeptidase complex / NADE modulates death signalling / neural retina development / luteolysis / execution phase of apoptosis / TP53 Regulates Transcription of Caspase Activators and Caspases / ectopic germ cell programmed cell death / extrinsic apoptotic signaling pathway in absence of ligand / positive regulation of apoptotic signaling pathway / DNA damage response, signal transduction by p53 class mediator / apoptotic signaling pathway / cellular response to mechanical stimulus / NOD1/2 Signaling Pathway / protein processing / intrinsic apoptotic signaling pathway in response to DNA damage / positive regulation of neuron apoptotic process / positive regulation of apoptotic process / protein domain specific binding / cysteine-type endopeptidase activity / apoptotic process / DNA damage response / negative regulation of apoptotic process / nucleolus / enzyme binding / identical protein binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.65 Å MOLECULAR REPLACEMENT / Resolution: 1.65 Å | ||||||
 Authors Authors | Schweizer, A. / Briand, C. / Grutter, M.G. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2003 Journal: J.Biol.Chem. / Year: 2003Title: Crystal structure of caspase-2, apical initiator of the intrinsic apoptotic pathway. Authors: Schweizer, A. / Briand, C. / Grutter, M.G. | ||||||
| History |
| ||||||
| Remark 999 | sequence Chains E and F represent a synthetic sequence that is commonly used for substrates and ...sequence Chains E and F represent a synthetic sequence that is commonly used for substrates and inhibitors for caspase-2 |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1pyo.cif.gz 1pyo.cif.gz | 125.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1pyo.ent.gz pdb1pyo.ent.gz | 97.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1pyo.json.gz 1pyo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/py/1pyo https://data.pdbj.org/pub/pdb/validation_reports/py/1pyo ftp://data.pdbj.org/pub/pdb/validation_reports/py/1pyo ftp://data.pdbj.org/pub/pdb/validation_reports/py/1pyo | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1cp3S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 18790.258 Da / Num. of mol.: 2 / Fragment: subunit p18, sequence database residues 151-316 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CASP2 OR ICH1 / Plasmid details: from Novagen / Plasmid: pET11d / Production host: Homo sapiens (human) / Gene: CASP2 OR ICH1 / Plasmid details: from Novagen / Plasmid: pET11d / Production host:  Strain (production host): BL21-CodonPlus(DE3)-RIL (Stratagene) References: UniProt: P42575, Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases #2: Protein | Mass: 11920.963 Da / Num. of mol.: 2 / Fragment: subunit p12, sequence database residues 331-435 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CASP2 OR ICH1 / Plasmid details: from Novagen / Plasmid: pET11d / Production host: Homo sapiens (human) / Gene: CASP2 OR ICH1 / Plasmid details: from Novagen / Plasmid: pET11d / Production host:  Strain (production host): BL21-CodonPlus(DE3)-RIL (Stratagene) References: UniProt: P42575, Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases #3: Protein/peptide | 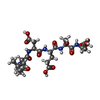  Type: Peptide-like / Class: Inhibitor / Mass: 589.594 Da / Num. of mol.: 2 / Source method: obtained synthetically / Details: synthetic, from Calbiochem Type: Peptide-like / Class: Inhibitor / Mass: 589.594 Da / Num. of mol.: 2 / Source method: obtained synthetically / Details: synthetic, from CalbiochemSource: (synth.)  References: UniProt: P36114, N-acetyl-L-leucyl-L-alpha-aspartyl-L-alpha-glutamyl-L-seryl-L-aspartic aldehyde #4: Water | ChemComp-HOH / | Compound details | THERE IS A COVALENT BOND BETWEEN ATOM SG OF CYS155 OF CHAIN A/C AND ATOM C OF ASA406 OF CHAIN E/F, ...THERE IS A COVALENT BOND BETWEEN ATOM SG OF CYS155 OF CHAIN A/C AND ATOM C OF ASA406 OF CHAIN E/F, FORMING THIOHEMIAC | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.4 Å3/Da / Density % sol: 49 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 7 Details: PEG 6000, MOPS, DTT, SUCROSE, pH 7.0, VAPOR DIFFUSION, SITTING DROP, temperature 277K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 4 ℃ / pH: 7.5 / Method: vapor diffusion, sitting drop | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 90 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 0.9184 Å / Beamline: X06SA / Wavelength: 0.9184 Å |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Feb 8, 2003 / Details: dynamically bendable mirror |
| Radiation | Monochromator: SI(111) crystal / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9184 Å / Relative weight: 1 |
| Reflection | Resolution: 1.65→25 Å / Num. all: 74129 / Num. obs: 74129 / % possible obs: 100 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Redundancy: 7.4 % / Biso Wilson estimate: 19.934 Å2 / Rsym value: 0.092 / Net I/σ(I): 21.14 |
| Reflection shell | Resolution: 1.65→1.71 Å / Mean I/σ(I) obs: 2.03 / Num. unique all: 7191 / Rsym value: 0.476 / % possible all: 100 |
| Reflection | *PLUS Rmerge(I) obs: 0.092 |
| Reflection shell | *PLUS % possible obs: 100 % / Rmerge(I) obs: 0.476 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1CP3 Resolution: 1.65→25 Å / Isotropic thermal model: anisotropic / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Displacement parameters | Biso mean: 23.121 Å2
| |||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.2 Å / Luzzati sigma a obs: 0.14 Å | |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.65→25 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.65→1.67 Å
| |||||||||||||||||||||||||
| Refinement | *PLUS Lowest resolution: 30 Å | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| |||||||||||||||||||||||||
| LS refinement shell | *PLUS Lowest resolution: 1.71 Å |
 Movie
Movie Controller
Controller












 PDBj
PDBj









