[English] 日本語
 Yorodumi
Yorodumi- PDB-1p06: STRUCTURE ANALYSIS OF SPECIFICITY. ALPHA-LYTIC PROTEASE COMPLEXES... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1p06 | ||||||
|---|---|---|---|---|---|---|---|
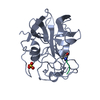
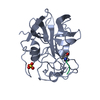
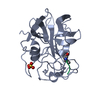
| Title | STRUCTURE ANALYSIS OF SPECIFICITY. ALPHA-LYTIC PROTEASE COMPLEXES WITH ANALOGUES OF REACTION INTERMEDIATES | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / HYDROLASE-HYDROLASE INHIBITOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationalpha-lytic endopeptidase / serine-type endopeptidase activity / proteolysis / extracellular region Similarity search - Function | ||||||
| Biological species |  Lysobacter enzymogenes (bacteria) Lysobacter enzymogenes (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.34 Å X-RAY DIFFRACTION / Resolution: 2.34 Å | ||||||
 Authors Authors | Bone, R. / Agard, D.A. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 1989 Journal: Biochemistry / Year: 1989Title: Structural analysis of specificity: alpha-lytic protease complexes with analogues of reaction intermediates. Authors: Bone, R. / Frank, D. / Kettner, C.A. / Agard, D.A. #1:  Journal: To be Published Journal: To be PublishedTitle: Structural Plasticity as a Determinant of Enzyme Specificity. Creating Broadly Specific Proteases Authors: Bone, R. / Silen, J.L. / Agard, D.A. #2:  Journal: Biochemistry / Year: 1988 Journal: Biochemistry / Year: 1988Title: Kinetic Properties of the Binding of Alpha-Lytic Protease to Peptide Boronic Acids Authors: Kettner, C.A. / Bone, R. / Agard, D.A. / Bachovchin, W.W. #3:  Journal: Biochemistry / Year: 1987 Journal: Biochemistry / Year: 1987Title: Serine Protease Mechanism. Structure of an Inhibitory Complex of Alpha-Lytic Protease and a Tightly Bound Peptide Boronic Acid Authors: Bone, R. / Shenvi, A.B. / Kettner, C.A. / Agard, D.A. #4:  Journal: J.Mol.Biol. / Year: 1985 Journal: J.Mol.Biol. / Year: 1985Title: Refined Structure of Alpha-Lytic Protease at 1.7 Angstroms Resolution. Analysis of Hydrogen Bonding and Solvent Structure Authors: Fujinaga, M. / Delbaere, L.T.J. / Brayer, G.D. / James, M.N.G. #5:  Journal: J.Mol.Biol. / Year: 1979 Journal: J.Mol.Biol. / Year: 1979Title: Molecular Structure of the Alpha-Lytic Protease from Myxobacter 495 at 2.8 Angstroms Resolution Authors: Brayer, G.D. / Delbaere, L.T.J. / James, M.N.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1p06.cif.gz 1p06.cif.gz | 54.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1p06.ent.gz pdb1p06.ent.gz | 37.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1p06.json.gz 1p06.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/p0/1p06 https://data.pdbj.org/pub/pdb/validation_reports/p0/1p06 ftp://data.pdbj.org/pub/pdb/validation_reports/p0/1p06 ftp://data.pdbj.org/pub/pdb/validation_reports/p0/1p06 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 19875.131 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Lysobacter enzymogenes (bacteria) / References: UniProt: P00778, alpha-lytic endopeptidase Lysobacter enzymogenes (bacteria) / References: UniProt: P00778, alpha-lytic endopeptidase |
|---|---|
| #2: Protein/peptide | |
| #3: Chemical | ChemComp-SO4 / |
| #4: Water | ChemComp-HOH / |
| Compound details | INHIBITORY PEPTIDE BORONIC ACIDS ARE PEPTIDE ANALOGS IN WHICH THE C-TERMINAL CARBOXYL GROUP HAS ...INHIBITORY |
| Has protein modification | Y |
| Nonpolymer details | INHIBITORY PEPTIDE BORONIC ACIDS ARE PEPTIDE ANALOGUES IN WHICH THE C-TERMINAL CARBOXY GROUP (COOH) ...INHIBITORY |
| Sequence details | CHAIN A RESIDUE NUMBERING IS DONE BY HOMOLOGY WITH CHYMOTRYPSIN FOR RESIDUES 15A - 244 AS DESCRIBED ...CHAIN A RESIDUE NUMBERING IS DONE BY HOMOLOGY WITH CHYMOTRYPS |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.53 Å3/Da / Density % sol: 51.45 % | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 6.8 / Method: vapor diffusion, hanging dropDetails: seeding, refer to Bone, R. et al (1987). Biochemistry, 27, 7609-7614. | ||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | Highest resolution: 2.34 Å |
| Reflection | *PLUS % possible obs: 86 % |
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Rfactor obs: 0.14 / Highest resolution: 2.34 Å Details: THE METHOXYSUCCINYL PORTION OF THE INHIBITOR WAS DISORDERED AND NO COORDINATES ARE INCLUDED FOR IT IN THIS ENTRY | ||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 2.34 Å
| ||||||||||||
| Refinement | *PLUS σ(I): 3 / Highest resolution: 2.34 Å / Rfactor obs: 0.14 | ||||||||||||
| Solvent computation | *PLUS | ||||||||||||
| Displacement parameters | *PLUS | ||||||||||||
| Refine LS restraints | *PLUS Type: p_bond_d / Dev ideal: 0.18 |
 Movie
Movie Controller
Controller












 PDBj
PDBj