+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1os2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Ternary enzyme-product-inhibitor complexes of human MMP12 | ||||||
 Components Components | Macrophage metalloelastase | ||||||
 Keywords Keywords | HYDROLASE / MATRIX METALLOPROTEINASE / HYDROXAMIC ACID / MMP12 / ELASTASE / COMPLEX (ELASTASE-INHIBITOR) / METALLO ELASTASE | ||||||
| Function / homology |  Function and homology information Function and homology informationmacrophage elastase / negative regulation of endothelial cell-matrix adhesion / bronchiole development / positive regulation of epithelial cell proliferation involved in wound healing / elastin catabolic process / regulation of defense response to virus by host / positive regulation of type I interferon-mediated signaling pathway / wound healing, spreading of epidermal cells / negative regulation of type I interferon-mediated signaling pathway / lung alveolus development ...macrophage elastase / negative regulation of endothelial cell-matrix adhesion / bronchiole development / positive regulation of epithelial cell proliferation involved in wound healing / elastin catabolic process / regulation of defense response to virus by host / positive regulation of type I interferon-mediated signaling pathway / wound healing, spreading of epidermal cells / negative regulation of type I interferon-mediated signaling pathway / lung alveolus development / response to amyloid-beta / Collagen degradation / collagen catabolic process / positive regulation of interferon-alpha production / extracellular matrix disassembly / core promoter sequence-specific DNA binding / collagen binding / Degradation of the extracellular matrix / extracellular matrix organization / extracellular matrix / metalloendopeptidase activity / cellular response to virus / protein import into nucleus / endopeptidase activity / sequence-specific DNA binding / serine-type endopeptidase activity / calcium ion binding / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / proteolysis / extracellular space / extracellular region / zinc ion binding / nucleus / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.15 Å MOLECULAR REPLACEMENT / Resolution: 2.15 Å | ||||||
 Authors Authors | Bertini, I. / Calderone, V. / Fragai, M. / Luchinat, C. / Mangani, S. / Terni, B. | ||||||
 Citation Citation |  Journal: Angew.Chem.Int.Ed.Engl. / Year: 2003 Journal: Angew.Chem.Int.Ed.Engl. / Year: 2003Title: X-ray Structures of Binary and Ternary Enzyme-Product-Inhibitor Complexes of Matrix Metalloproteinases Authors: Bertini, I. / Calderone, V. / Fragai, M. / Luchinat, C. / Mangani, S. / Terni, B. #1:  Journal: J.Mol.Biol. / Year: 2001 Journal: J.Mol.Biol. / Year: 2001Title: Substrate specificity determinants of human macrophage elastase (MMP-12) based on the 1.1 A crystal structure Authors: Lang, R. / Kocourek, A. / Braun, M. / Tschesche, H. / Huber, R. / Bode, W. / Maskos, K. | ||||||
| History |
| ||||||
| Remark 600 | HET GROUP TRIVIAL NAME: HAE IS ALSO KNOWN AS ACETOHYDROXAMIC ACID |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1os2.cif.gz 1os2.cif.gz | 217.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1os2.ent.gz pdb1os2.ent.gz | 173.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1os2.json.gz 1os2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1os2_validation.pdf.gz 1os2_validation.pdf.gz | 514.5 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1os2_full_validation.pdf.gz 1os2_full_validation.pdf.gz | 579.5 KB | Display | |
| Data in XML |  1os2_validation.xml.gz 1os2_validation.xml.gz | 50.4 KB | Display | |
| Data in CIF |  1os2_validation.cif.gz 1os2_validation.cif.gz | 67.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/os/1os2 https://data.pdbj.org/pub/pdb/validation_reports/os/1os2 ftp://data.pdbj.org/pub/pdb/validation_reports/os/1os2 ftp://data.pdbj.org/pub/pdb/validation_reports/os/1os2 | HTTPS FTP |
-Related structure data
| Related structure data |  1os9C  1jk3S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 | 
| ||||||||
| 6 | 
| ||||||||
| 7 | 
| ||||||||
| 8 | 
| ||||||||
| 9 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | The protein is monomeric in vivo, while there are six molecules in the crystal asymmetric unit. |
- Components
Components
-Protein , 1 types, 6 molecules ABCDEF
| #1: Protein | Mass: 18331.467 Da / Num. of mol.: 6 / Mutation: F171D Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: MMP12 OR HME / Production host: Homo sapiens (human) / Gene: MMP12 OR HME / Production host:  |
|---|
-Non-polymers , 6 types, 344 molecules 

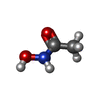








| #2: Chemical | ChemComp-ZN / #3: Chemical | ChemComp-CA / #4: Chemical | #5: Chemical | #6: Chemical | #7: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.71 Å3/Da / Density % sol: 54.28 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, sitting drop / pH: 8 Details: Tris, PEG6000, pH 8.0, VAPOR DIFFUSION, SITTING DROP, temperature 298K | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / Method: vapor diffusion | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID13 / Wavelength: 0.9322 Å / Beamline: ID13 / Wavelength: 0.9322 Å |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Dec 9, 2002 / Details: Si (111) mirrors |
| Radiation | Monochromator: Si (111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9322 Å / Relative weight: 1 |
| Reflection | Resolution: 2.15→107 Å / Num. all: 64964 / Num. obs: 64964 / % possible obs: 99.9 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 7.2 % / Biso Wilson estimate: 34 Å2 / Rmerge(I) obs: 0.076 / Rsym value: 0.076 / Net I/σ(I): 7.3 |
| Reflection shell | Resolution: 2.15→2.27 Å / Redundancy: 4.6 % / Rmerge(I) obs: 0.078 / Mean I/σ(I) obs: 1.9 / Num. unique all: 9418 / Rsym value: 0.078 / % possible all: 99.9 |
| Reflection | *PLUS Lowest resolution: 107 Å / % possible obs: 100 % / Num. measured all: 471410 |
| Reflection shell | *PLUS % possible obs: 100 % / Rmerge(I) obs: 0.076 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1JK3 Resolution: 2.15→69.01 Å / Cor.coef. Fo:Fc: 0.928 / Cor.coef. Fo:Fc free: 0.894 / SU B: 5.871 / SU ML: 0.153 / Isotropic thermal model: isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.244 / ESU R Free: 0.215 / Stereochemistry target values: MAXIMUM LIKELIHOOD
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 36.148 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.15→69.01 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.15→2.206 Å / Total num. of bins used: 20 /
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Num. reflection all: 64964 / Lowest resolution: 19.8 Å / Num. reflection Rfree: 3250 / % reflection Rfree: 5 % / Rfactor Rfree: 0.26 / Rfactor Rwork: 0.21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller












 PDBj
PDBj










