| 登録情報 | データベース: PDB / ID: 1nwb
|
|---|
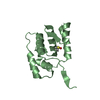
| タイトル | Solution NMR Structure of Protein AQ_1857 from Aquifex aeolicus: Northeast Structural Genomics Consortium Target QR6. |
|---|
 要素 要素 | Hypothetical protein AQ_1857 |
|---|
 キーワード キーワード | structural genomics / unknown function / QR6 / protein structure initiative / NESG / Reduced Dimensionality NMR / PSI / Northeast Structural Genomics Consortium |
|---|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報
iron-sulfur cluster assembly / 2 iron, 2 sulfur cluster binding / cytoplasm類似検索 - 分子機能 FeS cluster insertion, C-terminal, conserved site / Hypothetical hesB/yadR/yfhF family signature. / FeS cluster insertion protein / Hypothetical Protein Aq_1857; Chain: A; / HesB-like domain / FeS cluster biogenesis / HesB-like domain superfamily / Iron-sulphur cluster biosynthesis / Sandwich / Mainly Beta類似検索 - ドメイン・相同性 |
|---|
| 生物種 |   Aquifex aeolicus (バクテリア) Aquifex aeolicus (バクテリア) |
|---|
| 手法 | 溶液NMR / distance geometry torsion angle dynamics |
|---|
 データ登録者 データ登録者 | Xu, D. / Liu, G. / Xiao, R. / Acton, T. / Montelione, G.T. / Szyperski, T. / Northeast Structural Genomics Consortium (NESG) |
|---|
 引用 引用 |  ジャーナル: Proteins / 年: 2004 ジャーナル: Proteins / 年: 2004
タイトル: NMR structure of the hypothetical protein AQ-1857 encoded by the Y157 gene from Aquifex aeolicus reveals a novel protein fold.
著者: Xu, D. / Liu, G. / Xiao, R. / Acton, T. / Goldsmith-Fischman, S. / Honig, B. / Montelione, G.T. / Szyperski, T. |
|---|
| 履歴 | | 登録 | 2003年2月5日 | 登録サイト: RCSB / 処理サイト: RCSB |
|---|
| 改定 1.0 | 2003年6月3日 | Provider: repository / タイプ: Initial release |
|---|
| 改定 1.1 | 2008年4月29日 | Group: Version format compliance |
|---|
| 改定 1.2 | 2011年7月13日 | Group: Version format compliance |
|---|
| 改定 1.3 | 2022年2月23日 | Group: Database references / Derived calculations
カテゴリ: database_2 / pdbx_struct_assembly ...database_2 / pdbx_struct_assembly / pdbx_struct_oper_list / struct_ref_seq_dif
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_ref_seq_dif.details |
|---|
| 改定 1.4 | 2024年5月22日 | Group: Data collection / カテゴリ: chem_comp_atom / chem_comp_bond |
|---|
|
|---|
 データを開く
データを開く 基本情報
基本情報 要素
要素 キーワード
キーワード 機能・相同性情報
機能・相同性情報
 Aquifex aeolicus (バクテリア)
Aquifex aeolicus (バクテリア) データ登録者
データ登録者 引用
引用 ジャーナル: Proteins / 年: 2004
ジャーナル: Proteins / 年: 2004 構造の表示
構造の表示 Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク ダウンロード
ダウンロード 1nwb.cif.gz
1nwb.cif.gz PDBx/mmCIF形式
PDBx/mmCIF形式 pdb1nwb.ent.gz
pdb1nwb.ent.gz PDB形式
PDB形式 1nwb.json.gz
1nwb.json.gz PDBx/mmJSON形式
PDBx/mmJSON形式 その他のダウンロード
その他のダウンロード https://data.pdbj.org/pub/pdb/validation_reports/nw/1nwb
https://data.pdbj.org/pub/pdb/validation_reports/nw/1nwb ftp://data.pdbj.org/pub/pdb/validation_reports/nw/1nwb
ftp://data.pdbj.org/pub/pdb/validation_reports/nw/1nwb リンク
リンク 集合体
集合体
 要素
要素
 Aquifex aeolicus (バクテリア) / 遺伝子: AQ_1857 / プラスミド: pET21 / 生物種 (発現宿主): Escherichia coli / 発現宿主:
Aquifex aeolicus (バクテリア) / 遺伝子: AQ_1857 / プラスミド: pET21 / 生物種 (発現宿主): Escherichia coli / 発現宿主: 
 試料調製
試料調製 解析
解析 ムービー
ムービー コントローラー
コントローラー












 PDBj
PDBj HSQC
HSQC