[English] 日本語
 Yorodumi
Yorodumi- PDB-1kmf: NMR STRUCTURE OF HUMAN INSULIN MUTANT ILE-A2-ALLO-ILE, HIS-B10-AS... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1kmf | ||||||
|---|---|---|---|---|---|---|---|
| Title | NMR STRUCTURE OF HUMAN INSULIN MUTANT ILE-A2-ALLO-ILE, HIS-B10-ASP, PRO-B28-LYS, LYS-B29-PRO, 15 STRUCTURES | ||||||
 Components Components | (Insulin) x 2 | ||||||
 Keywords Keywords | HORMONE/GROWTH FACTOR / Hormone / Human insulin / Mutant / HORMONE-GROWTH FACTOR COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of glycogen catabolic process / positive regulation of nitric oxide mediated signal transduction / negative regulation of fatty acid metabolic process / negative regulation of feeding behavior / Signaling by Insulin receptor / IRS activation / regulation of protein secretion / Insulin processing / positive regulation of peptide hormone secretion / positive regulation of respiratory burst ...negative regulation of glycogen catabolic process / positive regulation of nitric oxide mediated signal transduction / negative regulation of fatty acid metabolic process / negative regulation of feeding behavior / Signaling by Insulin receptor / IRS activation / regulation of protein secretion / Insulin processing / positive regulation of peptide hormone secretion / positive regulation of respiratory burst / negative regulation of acute inflammatory response / Regulation of gene expression in beta cells / alpha-beta T cell activation / positive regulation of dendritic spine maintenance / Synthesis, secretion, and deacylation of Ghrelin / negative regulation of protein secretion / negative regulation of gluconeogenesis / positive regulation of glycogen biosynthetic process / fatty acid homeostasis / Signal attenuation / positive regulation of insulin receptor signaling pathway / negative regulation of respiratory burst involved in inflammatory response / FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes / negative regulation of lipid catabolic process / positive regulation of lipid biosynthetic process / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / regulation of protein localization to plasma membrane / nitric oxide-cGMP-mediated signaling / transport vesicle / COPI-mediated anterograde transport / positive regulation of nitric-oxide synthase activity / Insulin receptor recycling / negative regulation of reactive oxygen species biosynthetic process / positive regulation of brown fat cell differentiation / insulin-like growth factor receptor binding / NPAS4 regulates expression of target genes / neuron projection maintenance / endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of mitotic nuclear division / Insulin receptor signalling cascade / positive regulation of cytokine production / positive regulation of glycolytic process / endosome lumen / acute-phase response / positive regulation of long-term synaptic potentiation / positive regulation of protein secretion / positive regulation of D-glucose import across plasma membrane / insulin receptor binding / positive regulation of cell differentiation / Regulation of insulin secretion / wound healing / positive regulation of neuron projection development / hormone activity / regulation of synaptic plasticity / negative regulation of protein catabolic process / positive regulation of protein localization to nucleus / Golgi lumen / vasodilation / cognition / glucose metabolic process / insulin receptor signaling pathway / cell-cell signaling / glucose homeostasis / regulation of protein localization / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / positive regulation of cell growth / protease binding / secretory granule lumen / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / positive regulation of canonical NF-kappaB signal transduction / positive regulation of MAPK cascade / positive regulation of cell migration / G protein-coupled receptor signaling pathway / endoplasmic reticulum lumen / receptor ligand activity / Amyloid fiber formation / Golgi membrane / negative regulation of gene expression / positive regulation of cell population proliferation / positive regulation of gene expression / regulation of DNA-templated transcription / extracellular space / extracellular region / identical protein binding Similarity search - Function | ||||||
| Method | SOLUTION NMR / Distance geometry, simulated annealing | ||||||
 Authors Authors | Xu, B. / Hua, Q.X. / Nakagawa, S.H. / Jia, W. / Chu, Y.C. / Katsoyannis, P.G. / Weiss, M.A. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2002 Journal: J.Mol.Biol. / Year: 2002Title: Chiral mutagenesis of insulin's hidden receptor-binding surface: structure of an allo-isoleucine(A2) analogue. Authors: Xu, B. / Hua, Q.X. / Nakagawa, S.H. / Jia, W. / Chu, Y.C. / Katsoyannis, P.G. / Weiss, M.A. #1:  Journal: Biochemistry / Year: 1984 Journal: Biochemistry / Year: 1984Title: Critical Role of the A2 Amino Acid Residue in the Biological Activity of Insulin: [2-Glycine-A]- and [2-Alanine-A]insulins Authors: Kitagawa, K. / Ogawa, H. / Burke, G.T. / Chanley, J.D. / Katsoyannis, P.G. #2:  Journal: Biochemistry / Year: 1992 Journal: Biochemistry / Year: 1992Title: Importance of Aliphatic Side-Chain Structure at Positions 2 and 3 of the Insulin A Chain in Insulin-Receptor Interactions Authors: Nakagawa, S.H. / Tager, H.S. #3:  Journal: J.Mol.Biol. / Year: 1996 Journal: J.Mol.Biol. / Year: 1996Title: Mapping the Functional Surface of Insulin by Design: Structure and Function of a Novel A-Chain Analogue Authors: Hua, Q.X. / Hu, S.Q. / Frank, B.H. / Jia, W. / Chu, Y.C. / Wang, S.H. / Burke, G.T. / Katsoyannis, P.G. / Weiss, M.A. #4:  Journal: Protein Sci. / Year: 2002 Journal: Protein Sci. / Year: 2002Title: A Cavity-Forming Mutation in Insulin Induces Segmental Unfolding of a Surrounding alpha-Helix Authors: Xu, B. / Hua, Q.X. / Nakagawa, S.H. / Jia, W. / Chu, Y.C. / Katsoyannis, P.G. / Weiss, M.A. | ||||||
| History |
| ||||||
| Remark 650 | HELIX DETERMINATION METHOD: AUTHOR |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1kmf.cif.gz 1kmf.cif.gz | 234.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1kmf.ent.gz pdb1kmf.ent.gz | 193.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1kmf.json.gz 1kmf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/km/1kmf https://data.pdbj.org/pub/pdb/validation_reports/km/1kmf ftp://data.pdbj.org/pub/pdb/validation_reports/km/1kmf ftp://data.pdbj.org/pub/pdb/validation_reports/km/1kmf | HTTPS FTP |
|---|
-Related structure data
| Related structure data | |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 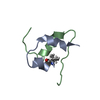
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 2383.698 Da / Num. of mol.: 1 / Mutation: I2(IIL) / Source method: obtained synthetically Details: The peptide was chemically synthesized. The sequence of the peptide is naturally found in Homo sapiens (human). References: UniProt: P01308 |
|---|---|
| #2: Protein/peptide | Mass: 3410.894 Da / Num. of mol.: 1 / Mutation: H10D, P28K, K29P / Source method: obtained synthetically Details: The peptide was chemically synthesized. The sequence of the peptide is naturally found in Homo sapiens (human). References: UniProt: P01308 |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||||||||||||||
| NMR details | Text: This structure was determined using standard 2D homonuclear techniques. |
- Sample preparation
Sample preparation
| Details |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample conditions |
| ||||||||||||
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| NMR spectrometer | Type: Varian INOVA / Manufacturer: Varian / Model: INOVA / Field strength: 600 MHz |
- Processing
Processing
| NMR software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: Distance geometry, simulated annealing / Software ordinal: 1 Details: The structure is based on a total of 599 restraints, 542 are NOE-derived distance constraints, 38 dihedral angle restraints, 19 distance restraints from hydrogen bonds. | ||||||||||||
| NMR representative | Selection criteria: closest to the average | ||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 50 / Conformers submitted total number: 15 |
 Movie
Movie Controller
Controller












 PDBj
PDBj

















 X-PLOR
X-PLOR