[English] 日本語
 Yorodumi
Yorodumi- PDB-1kcn: Structure of e109 Zeta Peptide, an Antagonist of the High-Affinit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1kcn | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of e109 Zeta Peptide, an Antagonist of the High-Affinity IgE Receptor | ||||||
 Components Components | e109 zeta peptide | ||||||
 Keywords Keywords | PROTEIN BINDING / disulfide-bonded / helical / "zeta" structure | ||||||
| Method | SOLUTION NMR / hybrid distance geometry, simulated annealing, then further refined by restrained molecular dynamics | ||||||
 Authors Authors | Nakamura, G.R. / Reynolds, M.E. / Chen, Y.M. / Starovasnik, M.A. / Lowman, H.B. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2002 Journal: Proc.Natl.Acad.Sci.USA / Year: 2002Title: Stable "zeta" peptides that act as potent antagonists of the high-affinity IgE receptor. Authors: Nakamura, G.R. / Reynolds, M.E. / Chen, Y.M. / Starovasnik, M.A. / Lowman, H.B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1kcn.cif.gz 1kcn.cif.gz | 106.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1kcn.ent.gz pdb1kcn.ent.gz | 73.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1kcn.json.gz 1kcn.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/kc/1kcn https://data.pdbj.org/pub/pdb/validation_reports/kc/1kcn ftp://data.pdbj.org/pub/pdb/validation_reports/kc/1kcn ftp://data.pdbj.org/pub/pdb/validation_reports/kc/1kcn | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 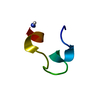
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 2143.615 Da / Num. of mol.: 1 / Source method: obtained synthetically Details: 'solid-phase peptide synthesis of a novel peptide based on a naive phage-peptide library that was sorted for binding to the high-affinity IGE receptor' |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||||||||||||||
| NMR details | Text: This structure was determined using standard 2D homonuclear techniques. 3JHNHA WERE OBTAINED BY FITTING LORENTZIAN LINES TO THE ANTIPHASE DOUBLETS OF HN-HA PEAKS IN A 2QF-COSY SPECTRUM ...Text: This structure was determined using standard 2D homonuclear techniques. 3JHNHA WERE OBTAINED BY FITTING LORENTZIAN LINES TO THE ANTIPHASE DOUBLETS OF HN-HA PEAKS IN A 2QF-COSY SPECTRUM PROCESSED TO HIGH DIGITAL RESOLUTION IN F2. 3JHAHB WERE EXTRACTED FROM A COSY-35 SPECTRUM ACQUIRED IN D2O |
- Sample preparation
Sample preparation
| Details |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample conditions | pH: 5.4 / Pressure: ambient / Temperature: 288 K | |||||||||
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| NMR spectrometer | Type: Bruker DRX / Manufacturer: Bruker / Model: DRX / Field strength: 500 MHz |
- Processing
Processing
| NMR software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: hybrid distance geometry, simulated annealing, then further refined by restrained molecular dynamics Software ordinal: 1 Details: The structures are based on a total of 101 NOE-derived distance restraints, 18 dihedral angle restraints, and 12 distant restraints from 6 hydrogen bonds. | ||||||||||||||||||||
| NMR representative | Selection criteria: closest to the average | ||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with acceptable covalent geometry,structures with the least restraint violations Conformers calculated total number: 50 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller








 PDBj
PDBj