[English] 日本語
 Yorodumi
Yorodumi- PDB-1jj8: Testing the Water-Mediated HIN Recombinase DNA Recognition by Sys... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1jj8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Testing the Water-Mediated HIN Recombinase DNA Recognition by Systematic Mutations | ||||||
 Components Components |
| ||||||
 Keywords Keywords | DNA BINDING PROTEIN/DNA / WATER-MEDIATED RECOGNITION / PROTEIN-DNA COMPLEX / HIN RECOMBINASE / I4 FORM 2 / DNA BINDING PROTEIN-DNA COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationDNA strand exchange activity / DNA integration / DNA recombination / DNA binding Similarity search - Function | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SIRAS / Resolution: 2.75 Å SIRAS / Resolution: 2.75 Å | ||||||
 Authors Authors | Chiu, T.K. / Sohn, C. / Johnson, R.C. / Dickerson, R.E. | ||||||
 Citation Citation |  Journal: EMBO J. / Year: 2002 Journal: EMBO J. / Year: 2002Title: Testing water-mediated DNA recognition by the Hin recombinase. Authors: Chiu, T.K. / Sohn, C. / Dickerson, R.E. / Johnson, R.C. #1:  Journal: Thesis / Year: 2001 Journal: Thesis / Year: 2001Title: How Hin Recombinase, FIS and Cations Bind DNA. Chapter 4. Water-Mediated Sequence-Specific Recognition by Hin Recombinase. Authors: Chiu, T.K. #2:  Journal: Science / Year: 1994 Journal: Science / Year: 1994Title: Hin recombinase bound to DNA: the origin of specificity in major and minor groove interactions. Authors: Feng, J.A. / Johnson, R.C. / Dickerson, R.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1jj8.cif.gz 1jj8.cif.gz | 39.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1jj8.ent.gz pdb1jj8.ent.gz | 24.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1jj8.json.gz 1jj8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jj/1jj8 https://data.pdbj.org/pub/pdb/validation_reports/jj/1jj8 ftp://data.pdbj.org/pub/pdb/validation_reports/jj/1jj8 ftp://data.pdbj.org/pub/pdb/validation_reports/jj/1jj8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1ijwC  1jj6C  1jkoC  1jkpC  1jkqC  1jkrC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 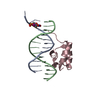
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: DNA chain | Mass: 4436.707 Da / Num. of mol.: 1 / Source method: obtained synthetically |
|---|---|
| #2: DNA chain | Mass: 4231.806 Da / Num. of mol.: 1 / Source method: obtained synthetically |
| #3: Protein | Mass: 6047.051 Da / Num. of mol.: 1 / Fragment: RESIDUES 139 TO 190 / Source method: obtained synthetically / Details: SYNTHETIC PEPTIDE / References: UniProt: P03013 |
| #4: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.423 Å3/Da / Density % sol: 47.27 % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Method: vapor diffusion, hanging drop / pH: 4.6 Details: 0.08 MM DNA, 0.04 MM HIN, 25 MM NA ACETATE (PH 4.6), 25 MM MGCL2, 8 MM NACL, 6.3% V/V PEG400, AND 1.25 MM NA CACODYLATE. RESERVOIR SOLUTION CONTAINS 100 MM NA ACETATE (PH 4.6), 100 MM MGCL2, ...Details: 0.08 MM DNA, 0.04 MM HIN, 25 MM NA ACETATE (PH 4.6), 25 MM MGCL2, 8 MM NACL, 6.3% V/V PEG400, AND 1.25 MM NA CACODYLATE. RESERVOIR SOLUTION CONTAINS 100 MM NA ACETATE (PH 4.6), 100 MM MGCL2, AND 25% PEG400. CONCENTRATION OF PEG400 IN RESERVOIR SOLUTION WAS INCREASED IN 5% INCREMENTS TO 35%. CONDITIONS FOR NATIVE ARE THE SAME EXCEPT THE CONCENTRATION OF MGCL2 IS 5 TIMES SMALLER., pH 4.60, VAPOR DIFFUSION, HANGING DROP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 21 ℃ / pH: 4.6 / Method: vapor diffusion, sitting drop | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Wavelength: 1.543 ROTATING ANODE / Wavelength: 1.543 |
| Detector | Type: RIGAKU / Detector: IMAGE PLATE / Date: Sep 1, 1995 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.543 Å / Relative weight: 1 |
| Reflection | Resolution: 2.75→25 Å / Num. obs: 3765 / % possible obs: 96.49 % / Observed criterion σ(I): 1 / Redundancy: 15 % / Biso Wilson estimate: 44 Å2 / Rsym value: 0.081 / Net I/σ(I): 19.6 |
| Reflection shell | Resolution: 2.75→2.87 Å / Redundancy: 4.2 % / Mean I/σ(I) obs: 4.5 / Rsym value: 0.267 / % possible all: 91.64 |
| Reflection | *PLUS % possible obs: 96.5 % / Redundancy: 15 % / Rmerge(I) obs: 0.081 |
| Reflection shell | *PLUS % possible obs: 91.6 % / Rmerge(I) obs: 0.222 / Mean I/σ(I) obs: 4.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SIRAS SIRASStarting model: SIRAS PHASES Resolution: 2.75→25 Å / Data cutoff high rms absF: 10000 / Isotropic thermal model: ANISOTROPIC_FIXED_ISOTROPIC / σ(F): 0 / Stereochemistry target values: MLF
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Bsol: 18.88 Å2 / ksol: 0.28 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 45 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.75→25 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.75→2.87 Å / Total num. of bins used: 8
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS % reflection Rfree: 10 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor obs: 0.353 |
 Movie
Movie Controller
Controller












 PDBj
PDBj







































