[English] 日本語
 Yorodumi
Yorodumi- PDB-1ivp: THE CRYSTALLOGRAPHIC STRUCTURE OF THE PROTEASE FROM HUMAN IMMUNOD... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ivp | ||||||
|---|---|---|---|---|---|---|---|
| Title | THE CRYSTALLOGRAPHIC STRUCTURE OF THE PROTEASE FROM HUMAN IMMUNODEFICIENCY VIRUS TYPE 2 WITH TWO SYNTHETIC PEPTIDIC TRANSITION STATE ANALOG INHIBITORS | ||||||
 Components Components | HIV-2 PROTEASE | ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR / ACID PROTEINASE / HYDROLASE-HYDROLASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationHIV-2 retropepsin / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / host multivesicular body / DNA integration / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / viral penetration into host nucleus ...HIV-2 retropepsin / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / host multivesicular body / DNA integration / viral genome integration into host DNA / RNA-directed DNA polymerase / establishment of integrated proviral latency / viral penetration into host nucleus / RNA stem-loop binding / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / lipid binding / symbiont entry into host cell / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding / membrane Similarity search - Function | ||||||
| Biological species |  Human immunodeficiency virus 2 Human immunodeficiency virus 2 | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.5 Å X-RAY DIFFRACTION / Resolution: 2.5 Å | ||||||
 Authors Authors | Mulichak, A.M. / Watenpaugh, K.D. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 1993 Journal: J.Biol.Chem. / Year: 1993Title: The crystallographic structure of the protease from human immunodeficiency virus type 2 with two synthetic peptidic transition state analog inhibitors. Authors: Mulichak, A.M. / Hui, J.O. / Tomasselli, A.G. / Heinrikson, R.L. / Curry, K.A. / Tomich, C.S. / Thaisrivongs, S. / Sawyer, T.K. / Watenpaugh, K.D. | ||||||
| History |
|
- Structure visualization
Structure visualization
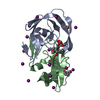
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ivp.cif.gz 1ivp.cif.gz | 52.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ivp.ent.gz pdb1ivp.ent.gz | 36.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ivp.json.gz 1ivp.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1ivp_validation.pdf.gz 1ivp_validation.pdf.gz | 472.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1ivp_full_validation.pdf.gz 1ivp_full_validation.pdf.gz | 490.2 KB | Display | |
| Data in XML |  1ivp_validation.xml.gz 1ivp_validation.xml.gz | 9.2 KB | Display | |
| Data in CIF |  1ivp_validation.cif.gz 1ivp_validation.cif.gz | 12.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/iv/1ivp https://data.pdbj.org/pub/pdb/validation_reports/iv/1ivp ftp://data.pdbj.org/pub/pdb/validation_reports/iv/1ivp ftp://data.pdbj.org/pub/pdb/validation_reports/iv/1ivp | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: SEE REMARK 5 ABOVE. / 2: SEE REMARK 6 ABOVE. | ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (0.2141, 0.9365, -0.2776), Vector: Details | THE NON-CRYSTALLOGRAPHIC C(2) SYMMETRY RELATING THE TWO MONOMERS OF THE PROTEASE DIMER IS GIVEN ON THE MTRIX RECORDS BELOW. THIS TRANSFORMATION WILL YIELD APPROXIMATE COORDINATES FOR CHAIN B WHEN APPLIED TO CHAIN A. | |
- Components
Components
| #1: Protein | Mass: 10712.315 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Human immunodeficiency virus 2 / Genus: Lentivirus / Production host: Human immunodeficiency virus 2 / Genus: Lentivirus / Production host:  #2: Chemical | ChemComp-1ZK / | #3: Water | ChemComp-HOH / | Nonpolymer details | COMPONENT CAV 3 OF THE INHIBITOR 1ZK B 100 IS THE DIPEPTIDE ISOSTERE CYCLOHEXYL ALANINE PSI(CHOH- ...COMPONENT CAV 3 OF THE INHIBITOR 1ZK B 100 IS THE DIPEPTIDE ISOSTERE CYCLOHEXYL | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.27 Å3/Da / Density % sol: 45.91 % | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS pH: 7 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 2.5 Å / Num. obs: 5285 / % possible obs: 86 % / Rmerge F obs: 0.095 / Num. measured all: 20722 |
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Rfactor obs: 0.197 / Highest resolution: 2.5 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 2.5 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.5 Å / Num. reflection obs: 5563 / Rfactor obs: 0.197 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS Biso mean: 18 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller














 PDBj
PDBj