[English] 日本語
 Yorodumi
Yorodumi- PDB-1hfo: The Structure of the Macrophage Migration Inhibitory Factor from ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1hfo | ||||||
|---|---|---|---|---|---|---|---|
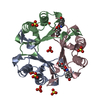
| Title | The Structure of the Macrophage Migration Inhibitory Factor from Trichinella Spiralis. | ||||||
 Components Components | MIGRATION INHIBITORY FACTOR | ||||||
 Keywords Keywords | TAUTOMERASE | ||||||
| Function / homology |  Function and homology information Function and homology informationdopachrome isomerase activity / phenylpyruvate tautomerase / L-dopachrome isomerase / phenylpyruvate tautomerase activity / cytokine activity / extracellular space Similarity search - Function | ||||||
| Biological species |  TRICHINELLA SPIRALIS (invertebrata) TRICHINELLA SPIRALIS (invertebrata) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.65 Å MOLECULAR REPLACEMENT / Resolution: 1.65 Å | ||||||
 Authors Authors | Roe, S.M. / Meyer, D.J. | ||||||
 Citation Citation |  Journal: Biochem.J. / Year: 2001 Journal: Biochem.J. / Year: 2001Title: Macrophage Migration Inhibitory Factor of the Parasitic Nematode Trichinella Spiralis Authors: Tan, T.H. / Edgerton, S.A. / Kumari, R. / Mcalister, M.S. / Rowe, S.M. / Nagl, S. / Pearl, L.H. / Selkirk, M.E. / Bianco, A.E. / Totty, N.F. / Engwerda, C. / Gray, C.A. / Meyer, D.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1hfo.cif.gz 1hfo.cif.gz | 158.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1hfo.ent.gz pdb1hfo.ent.gz | 126.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1hfo.json.gz 1hfo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1hfo_validation.pdf.gz 1hfo_validation.pdf.gz | 461 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1hfo_full_validation.pdf.gz 1hfo_full_validation.pdf.gz | 470.6 KB | Display | |
| Data in XML |  1hfo_validation.xml.gz 1hfo_validation.xml.gz | 38.7 KB | Display | |
| Data in CIF |  1hfo_validation.cif.gz 1hfo_validation.cif.gz | 57.7 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hf/1hfo https://data.pdbj.org/pub/pdb/validation_reports/hf/1hfo ftp://data.pdbj.org/pub/pdb/validation_reports/hf/1hfo ftp://data.pdbj.org/pub/pdb/validation_reports/hf/1hfo | HTTPS FTP |
-Related structure data
| Related structure data |  1mifS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | BIOLOGICAL_UNIT: HOMOTRIMER |
- Components
Components
| #1: Protein | Mass: 12176.610 Da / Num. of mol.: 6 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  TRICHINELLA SPIRALIS (invertebrata) / Production host: TRICHINELLA SPIRALIS (invertebrata) / Production host:  #2: Water | ChemComp-HOH / | Sequence details | MASS SPEC ANALYSIS AND THE CRYSTAL STRUCTURE (AT 1.65A) UNAMBIGUOS | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2 Å3/Da / Density % sol: 40.5 % | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Method: vapor diffusion, hanging drop / pH: 4.6 Details: CRYSTALS GROWN BY HANGING DROP METHOD. WELL CONTAINED 400-600 AMMONIUM ACETATE, 30-36% PEG4K, 100MM SODIUM ACETATE PH4.6. DROP CONTAINED 1:1 MIX OF WELL AND 16.5MG/ML TSMIF IN MINIMAL BUFFER., pH 4.60 | |||||||||||||||||||||||||
| Crystal grow | *PLUS Method: batch method | |||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-3 / Wavelength: 0.93 / Beamline: ID14-3 / Wavelength: 0.93 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Nov 15, 1999 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.93 Å / Relative weight: 1 |
| Reflection | Resolution: 1.65→28 Å / Num. obs: 72915 / % possible obs: 97.6 % / Redundancy: 2.3 % / Biso Wilson estimate: 26.9 Å2 / Rsym value: 0.057 / Net I/σ(I): 3.6 |
| Reflection shell | Resolution: 1.65→1.71 Å / Redundancy: 2.2 % / Mean I/σ(I) obs: 2.5 / Rsym value: 0.281 / % possible all: 97.6 |
| Reflection | *PLUS Lowest resolution: 28 Å / Num. measured all: 163895 / Rmerge(I) obs: 0.057 |
| Reflection shell | *PLUS % possible obs: 97.6 % / Num. unique obs: 7060 / Num. measured obs: 16174 / Rmerge(I) obs: 0.281 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1MIF Resolution: 1.65→28.33 Å / Rfactor Rfree error: 0.005 / Data cutoff high absF: 1356759.35 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 Details: NCS USED INITIALLY, BUT REMOVED IN FINAL STAGES. THERE IS A LARGE AREA OF UNMODELLED ELECTRON DENSITY AT THE N-TERMINUS OF EACH CHAIN. IT IS UNKNOWN WHAT THIS MAY BE, AND IS ASSUMED TO HAVE ...Details: NCS USED INITIALLY, BUT REMOVED IN FINAL STAGES. THERE IS A LARGE AREA OF UNMODELLED ELECTRON DENSITY AT THE N-TERMINUS OF EACH CHAIN. IT IS UNKNOWN WHAT THIS MAY BE, AND IS ASSUMED TO HAVE COPURIFIED WITH THE PROTEIN. IT SITS AT THE SITE OF TAUTOMERASE ACTIVITY.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 67.3598 Å2 / ksol: 0.309311 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 31.3 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.65→28.33 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.65→1.75 Å / Rfactor Rfree error: 0.014 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rwork: 0.31 |
 Movie
Movie Controller
Controller











 PDBj
PDBj
