[English] 日本語
 Yorodumi
Yorodumi- PDB-1gq4: STRUCTURAL DETERMINANTS OF THE NHERF INTERACTION WITH BETA2AR AND... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1gq4 | ||||||
|---|---|---|---|---|---|---|---|
| Title | STRUCTURAL DETERMINANTS OF THE NHERF INTERACTION WITH BETA2AR AND PDGFR | ||||||
 Components Components | EZRIN-RADIXIN-MOESIN BINDING PHOSPHOPROTEIN-50 | ||||||
 Keywords Keywords | SIGNALING PROTEIN / PDZ / BETA2-ADRENERGIC RECEPTOR / PDGFR / NHERF / COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationrenal phosphate ion absorption / regulation of renal phosphate excretion / renal sodium ion transport / type 2 metabotropic glutamate receptor binding / dopamine receptor binding / glutathione transport / cerebrospinal fluid circulation / negative regulation of sodium ion transport / microvillus assembly / maintenance of epithelial cell apical/basal polarity ...renal phosphate ion absorption / regulation of renal phosphate excretion / renal sodium ion transport / type 2 metabotropic glutamate receptor binding / dopamine receptor binding / glutathione transport / cerebrospinal fluid circulation / negative regulation of sodium ion transport / microvillus assembly / maintenance of epithelial cell apical/basal polarity / regulation of protein kinase activity / import across plasma membrane / bile acid secretion / stereocilium tip / gamma-aminobutyric acid import / plasma membrane organization / phospholipase C-activating dopamine receptor signaling pathway / channel activator activity / gland morphogenesis / cilium organization / establishment of Golgi localization / fibroblast migration / chloride channel regulator activity / establishment of epithelial cell apical/basal polarity / plasma membrane protein complex / type 3 metabotropic glutamate receptor binding / negative regulation of fibroblast migration / intracellular phosphate ion homeostasis / positive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / negative regulation of smooth muscle contraction / auditory receptor cell stereocilium organization / negative regulation of mitotic cell cycle / AMPA selective glutamate receptor signaling pathway / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / norepinephrine binding / Adrenoceptors / beta-2 adrenergic receptor binding / positive regulation of lipophagy / growth factor receptor binding / negative regulation of platelet-derived growth factor receptor signaling pathway / negative regulation of G protein-coupled receptor signaling pathway / negative regulation of multicellular organism growth / nuclear migration / response to psychosocial stress / adrenergic receptor signaling pathway / endosome to lysosome transport / diet induced thermogenesis / renal absorption / microvillus membrane / regulation of cell size / microvillus / positive regulation of cAMP/PKA signal transduction / adenylate cyclase binding / smooth muscle contraction / bone resorption / potassium channel regulator activity / positive regulation of bone mineralization / neuronal dense core vesicle / phosphatase binding / transport across blood-brain barrier / intercellular bridge / regulation of sodium ion transport / adenylate cyclase-activating adrenergic receptor signaling pathway / positive regulation of intrinsic apoptotic signaling pathway / ruffle / protein-membrane adaptor activity / endomembrane system / brown fat cell differentiation / receptor-mediated endocytosis / negative regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / response to cold / morphogenesis of an epithelium / cell periphery / protein localization to plasma membrane / PDZ domain binding / filopodium / clathrin-coated endocytic vesicle membrane / brush border membrane / sensory perception of sound / negative regulation of canonical Wnt signaling pathway / beta-catenin binding / negative regulation of ERK1 and ERK2 cascade / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / cellular response to amyloid-beta / Wnt signaling pathway / mitotic spindle / adenylate cyclase-activating dopamine receptor signaling pathway / sperm midpiece / Cargo recognition for clathrin-mediated endocytosis / regulation of cell shape / actin cytoskeleton / positive regulation of cold-induced thermogenesis / amyloid-beta binding / Clathrin-mediated endocytosis / microtubule cytoskeleton / actin cytoskeleton organization / protein-containing complex assembly Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | ||||||
 Authors Authors | Karthikeyan, S. / Leung, T. / Ladias, J.A.A. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2002 Journal: J.Biol.Chem. / Year: 2002Title: Structural Determinants of the Na+/H+ Exchanger Regulatory Factor Interaction with the Beta 2 Adrenergic and Platelet-Derived Growth Factor Receptors Authors: Karthikeyan, S. / Leung, T. / Ladias, J.A.A. #1:  Journal: J.Mol.Biol. / Year: 2001 Journal: J.Mol.Biol. / Year: 2001Title: Crystal Structure of the Pdz1 Domain of Human Na+/ H+ Exchanger Regulatory Factor Provides Insights Into the Mechanism of Carboxyl-Terminal Leucine Recognition by Class I Pdz Domains Authors: Karthikeyan, S. / Leung, T. / Birrane, G. / Webster, G. / Ladias, J.A.A. #2:  Journal: J.Biol.Chem. / Year: 2001 Journal: J.Biol.Chem. / Year: 2001Title: Structural Basis of the Na+/H+ Exchanger Regulatory Factor Pdz1 Interaction with the Carboxyl-Terminal Region of the Cystic Fibrosis Transmembrane Conductance Regulator Authors: Karthikeyan, S. / Leung, T. / Ladias, J.A.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
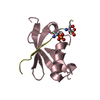
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1gq4.cif.gz 1gq4.cif.gz | 33 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1gq4.ent.gz pdb1gq4.ent.gz | 21.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1gq4.json.gz 1gq4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gq/1gq4 https://data.pdbj.org/pub/pdb/validation_reports/gq/1gq4 ftp://data.pdbj.org/pub/pdb/validation_reports/gq/1gq4 ftp://data.pdbj.org/pub/pdb/validation_reports/gq/1gq4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1gq5C  1i92S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 9842.304 Da / Num. of mol.: 1 / Fragment: PDZ1 DOMAIN, RESIDUES 11-94 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PGEX-2T / Production host: HOMO SAPIENS (human) / Plasmid: PGEX-2T / Production host:  | ||||
|---|---|---|---|---|---|
| #2: Chemical | ChemComp-CL / #3: Water | ChemComp-HOH / | Sequence details | THE N-TERMINAL MET, RESIDUE 10, IS A CLONING ARTIFACT. THE AMINO ACID RESIDUES 95-99 OF PDZ1 ...THE N-TERMINAL MET, RESIDUE 10, IS A CLONING ARTIFACT. THE AMINO ACID RESIDUES 95-99 OF PDZ1 CORRESPOND | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.44 Å3/Da / Density % sol: 49.6 % | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 4.24 / Details: CITRIC ACID, LITHIUM CHLORIDE, pH 4.24 | ||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ / Method: vapor diffusion, sitting drop | ||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 115 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU300 / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RU300 / Wavelength: 1.5418 |
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE / Date: Jun 29, 2001 / Details: MIRRORS |
| Radiation | Monochromator: NI FILTER / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→26.48 Å / Num. obs: 8091 / % possible obs: 99.8 % / Observed criterion σ(I): -3 / Redundancy: 5.4 % / Biso Wilson estimate: 18.73 Å2 / Rmerge(I) obs: 0.059 / Net I/σ(I): 23.4 |
| Reflection shell | Resolution: 1.9→1.97 Å / Redundancy: 4.9 % / Rmerge(I) obs: 0.194 / Mean I/σ(I) obs: 8.4 / % possible all: 99.7 |
| Reflection | *PLUS Highest resolution: 1.9 Å / Lowest resolution: 26.5 Å / Num. obs: 8069 / Num. measured all: 64231 |
| Reflection shell | *PLUS % possible obs: 99.7 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1I92 Resolution: 1.9→26.48 Å / Cor.coef. Fo:Fc: 0.955 / Cor.coef. Fo:Fc free: 0.947 / SU B: 6.177 / SU ML: 0.177 / Cross valid method: THROUGHOUT / ESU R: 0.138 / ESU R Free: 0.115 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 15.73 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.9→26.48 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj









