[English] 日本語
 Yorodumi
Yorodumi- PDB-1el9: COMPLEX OF MONOMERIC SARCOSINE OXIDASE WITH THE INHIBITOR [METHYL... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1el9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | COMPLEX OF MONOMERIC SARCOSINE OXIDASE WITH THE INHIBITOR [METHYLTHIO]ACETATE | ||||||
 Components Components | SARCOSINE OXIDE | ||||||
 Keywords Keywords | OXIDOREDUCTASE / flavoprotein / oxidase | ||||||
| Function / homology |  Function and homology information Function and homology informationsarcosine oxidase (formaldehyde-forming) / sarcosine oxidase activity / flavin adenine dinucleotide binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2 Å X-RAY DIFFRACTION / Resolution: 2 Å | ||||||
 Authors Authors | Wagner, M.A. / Trickey, P. / Chen, Z.-W. / Mathews, F.S. / Jorns, M.S. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2000 Journal: Biochemistry / Year: 2000Title: Monomeric sarcosine oxidase: 1. Flavin reactivity and active site binding determinants. Authors: Wagner, M.A. / Trickey, P. / Chen, Z.W. / Mathews, F.S. / Jorns, M.S. #1:  Journal: Structure / Year: 1999 Journal: Structure / Year: 1999Title: Monomeric Sarcosine Oxidase: Structure of a Covalently Flavinylated Amine Oxidizing Enzyme Authors: Trickey, P. / Wagner, M.A. / Jorns, M.S. / Mathews, F.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1el9.cif.gz 1el9.cif.gz | 179.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1el9.ent.gz pdb1el9.ent.gz | 140.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1el9.json.gz 1el9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/el/1el9 https://data.pdbj.org/pub/pdb/validation_reports/el/1el9 ftp://data.pdbj.org/pub/pdb/validation_reports/el/1el9 ftp://data.pdbj.org/pub/pdb/validation_reports/el/1el9 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1el5C  1el7C  1el8C  1eliC  1b3m C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Details | The biological assembly is monomer. |
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 43429.617 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P40859, sarcosine oxidase (formaldehyde-forming) |
|---|
-Non-polymers , 5 types, 561 molecules 


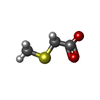





| #2: Chemical | | #3: Chemical | #4: Chemical | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.14 Å3/Da / Density % sol: 42.62 % Description: MAD phasing was not used to solve 1EL9 and 1ELI, but rather direct refinement as the crystals were isomorphous enough to the native crystals for phasing. Selenomethionine crystals were ...Description: MAD phasing was not used to solve 1EL9 and 1ELI, but rather direct refinement as the crystals were isomorphous enough to the native crystals for phasing. Selenomethionine crystals were used for the experiments since they were the only ones on hand at the time. Kinetic studies showed that the presence of selenomethionine had little effect. | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, sitting drop / pH: 7 Details: phosphate, tris-HCl, pH 7.0, VAPOR DIFFUSION, SITTING DROP, temperature 295.0K | ||||||||||||||||||||
| Crystal grow | *PLUS pH: 8 Details: drop consists of equal amounts of protein and reservoir solutions | ||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 |
| Detector | Type: RIGAKU RAXIS / Detector: IMAGE PLATE / Date: Jul 23, 1998 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2→500 Å / Num. all: 49552 / Num. obs: 43804 / % possible obs: 88.4 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.8 % / Biso Wilson estimate: 9.5 Å2 / Rmerge(I) obs: 0.088 / Net I/σ(I): 10.3 |
| Reflection shell | Resolution: 2→2.07 Å / Redundancy: 2.55 % / Rmerge(I) obs: 0.25 / Num. unique all: 2639 / % possible all: 53.6 |
| Reflection shell | *PLUS % possible obs: 53.6 % |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2→500 Å / σ(F): 0 / σ(I): 0 / Stereochemistry target values: ENGH & HUBER
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→500 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 0.9 / Classification: refinement | |||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor Rfree: 0.311 / Rfactor Rwork: 0.278 |
 Movie
Movie Controller
Controller














 PDBj
PDBj



