+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1b79 | ||||||
|---|---|---|---|---|---|---|---|
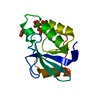
| Title | N-TERMINAL DOMAIN OF DNA REPLICATION PROTEIN DNAB | ||||||
 Components Components | DnaB Helicase | ||||||
 Keywords Keywords | HYDROLASE / HELICASE / HEXAMER / DNA REPLICATION | ||||||
| Function / homology |  Function and homology information Function and homology informationDnaB-DnaC complex / DnaB-DnaC-Rep-PriC complex / DnaB-DnaG complex / DnaB-DnaC-DnaT-PriA-PriC complex / DnaB-DnaC-DnaT-PriA-PriB complex / DNA helicase complex / primosome complex / DNA 5'-3' helicase / DNA replication, synthesis of primer / replisome ...DnaB-DnaC complex / DnaB-DnaC-Rep-PriC complex / DnaB-DnaG complex / DnaB-DnaC-DnaT-PriA-PriC complex / DnaB-DnaC-DnaT-PriA-PriB complex / DNA helicase complex / primosome complex / DNA 5'-3' helicase / DNA replication, synthesis of primer / replisome / response to ionizing radiation / replication fork processing / DNA replication initiation / DNA helicase activity / helicase activity / 5'-3' DNA helicase activity / DNA replication / ATP hydrolysis activity / DNA binding / ATP binding / identical protein binding / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MIR / Resolution: 2.3 Å MIR / Resolution: 2.3 Å | ||||||
 Authors Authors | Fass, D. / Bogden, C.E. / Berger, J.M. | ||||||
 Citation Citation |  Journal: Structure Fold.Des. / Year: 1999 Journal: Structure Fold.Des. / Year: 1999Title: Crystal structure of the N-terminal domain of the DnaB hexameric helicase. Authors: Fass, D. / Bogden, C.E. / Berger, J.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1b79.cif.gz 1b79.cif.gz | 89.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1b79.ent.gz pdb1b79.ent.gz | 70.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1b79.json.gz 1b79.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/b7/1b79 https://data.pdbj.org/pub/pdb/validation_reports/b7/1b79 ftp://data.pdbj.org/pub/pdb/validation_reports/b7/1b79 ftp://data.pdbj.org/pub/pdb/validation_reports/b7/1b79 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||
| 2 | 
| ||||||||||||||||
| 3 | 
| ||||||||||||||||
| 4 | 
| ||||||||||||||||
| Unit cell |
| ||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
| #1: Protein | Mass: 13222.717 Da / Num. of mol.: 4 / Fragment: N-TERMINAL DOMAIN Source method: isolated from a genetically manipulated source Details: hexamers not associated in crystal / Source: (gene. exp.)   References: UniProt: P0ACB0, Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides #2: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.36 Å3/Da / Density % sol: 53 % | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Method: vapor diffusion, hanging drop / pH: 6.9 / Details: pH 6.9, VAPOR DIFFUSION, HANGING DROP | ||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | Name: SODIUM CACODYLATE | ||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 20 ℃ | ||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 118 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X4A / Wavelength: 0.9667 / Beamline: X4A / Wavelength: 0.9667 |
| Detector | Date: Feb 15, 1998 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9667 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→20 Å / Num. obs: 21651 / % possible obs: 98.7 % / Redundancy: 3.4 % / Rsym value: 6.9 / Net I/σ(I): 9 |
| Reflection | *PLUS Rmerge(I) obs: 0.069 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MIR / Resolution: 2.3→20 Å / Rfactor Rfree error: 0.008 / Cross valid method: THROUGHOUT / σ(F): 2 MIR / Resolution: 2.3→20 Å / Rfactor Rfree error: 0.008 / Cross valid method: THROUGHOUT / σ(F): 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | NCS model details: RESTRAINTS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.3→2.4 Å / Rfactor Rfree error: 0.035 / Total num. of bins used: 8 /
|
 Movie
Movie Controller
Controller











 PDBj
PDBj

