[English] 日本語
 Yorodumi
Yorodumi- PDB-1amr: X-RAY CRYSTALLOGRAPHIC STUDY OF PYRIDOXAMINE 5'-PHOSPHATE-TYPE AS... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1amr | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-RAY CRYSTALLOGRAPHIC STUDY OF PYRIDOXAMINE 5'-PHOSPHATE-TYPE ASPARTATE AMINOTRANSFERASES FROM ESCHERICHIA COLI IN THREE FORMS | ||||||
 Components Components | ASPARTATE AMINOTRANSFERASE | ||||||
 Keywords Keywords | TRANSFERASE(AMINOTRANSFERASE) | ||||||
| Function / homology |  Function and homology information Function and homology informationL-phenylalanine biosynthetic process from chorismate via phenylpyruvate / L-tyrosine-2-oxoglutarate transaminase activity / L-phenylalanine biosynthetic process / aspartate transaminase / L-aspartate:2-oxoglutarate aminotransferase activity / pyridoxal phosphate binding / protein homodimerization activity / identical protein binding / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.1 Å X-RAY DIFFRACTION / Resolution: 2.1 Å | ||||||
 Authors Authors | Miyahara, I. / Hirotsu, K. / Hayashi, H. / Kagamiyama, H. | ||||||
 Citation Citation |  Journal: J.Biochem.(Tokyo) / Year: 1994 Journal: J.Biochem.(Tokyo) / Year: 1994Title: X-ray crystallographic study of pyridoxamine 5'-phosphate-type aspartate aminotransferases from Escherichia coli in three forms. Authors: Miyahara, I. / Hirotsu, K. / Hayashi, H. / Kagamiyama, H. #1:  Journal: To be Published Journal: To be PublishedTitle: X-Ray Crystallographic Study of Pyridoxal 5'-Phosphate-Type Aspartate Aminotransferase from Esherichia Coli in Open and Closed Form Authors: Okamoto, A. / Higuchi, T. / Hirotsu, K. / Kuramitsu, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1amr.cif.gz 1amr.cif.gz | 95 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1amr.ent.gz pdb1amr.ent.gz | 71.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1amr.json.gz 1amr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/am/1amr https://data.pdbj.org/pub/pdb/validation_reports/am/1amr ftp://data.pdbj.org/pub/pdb/validation_reports/am/1amr ftp://data.pdbj.org/pub/pdb/validation_reports/am/1amr | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: CIS PROLINE - PRO 138 / 2: CIS PROLINE - PRO 195 | ||||||||
| Details | THE FUNCTIONAL DIMER CAN BE GENERATED BY APPLYING THE SYMMETRY OPERATOR (X, -Y, -Z) TO THE ATOMIC COORDINATES PRESENTED IN THIS ENTRY. |
- Components
Components
| #1: Protein | Mass: 43619.215 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
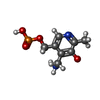
| #2: Chemical | ChemComp-PMP / |
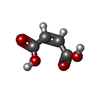
| #3: Chemical | ChemComp-MAE / |
| #4: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.05 Å3/Da / Density % sol: 59.71 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 20 ℃ / pH: 7 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 1.8 Å / Num. obs: 30641 / Num. measured all: 98558 / Rmerge(I) obs: 0.0682 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.1→10 Å / σ(F): 3 /
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→10 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor obs: 0.194 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: x_angle_d / Dev ideal: 1.787 |
 Movie
Movie Controller
Controller














 PDBj
PDBj