+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1al1 | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF ALPHA1: IMPLICATIONS FOR PROTEIN DESIGN | ||||||
 Components Components | ALPHA HELIX PEPTIDE: ELLKKLLEELKG | ||||||
 Keywords Keywords | SYNTHETIC PROTEIN MODEL | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.7 Å X-RAY DIFFRACTION / Resolution: 2.7 Å | ||||||
 Authors Authors | Hill, C.P. / Anderson, D.H. / Wesson, L. / Degrado, W.F. / Eisenberg, D. | ||||||
 Citation Citation |  Journal: Science / Year: 1990 Journal: Science / Year: 1990Title: Crystal structure of alpha 1: implications for protein design. Authors: Hill, C.P. / Anderson, D.H. / Wesson, L. / DeGrado, W.F. / Eisenberg, D. #1:  Journal: Proteins / Year: 1986 Journal: Proteins / Year: 1986Title: The Design, Synthesis, and Crystallization of an Alpha-Helical Peptide Authors: Eisenberg, D. / Wilcox, W. / Eshita, S.M. / Pryciak, P.M. / Ho, S.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1al1.cif.gz 1al1.cif.gz | 9.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1al1.ent.gz pdb1al1.ent.gz | 7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1al1.json.gz 1al1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1al1_validation.pdf.gz 1al1_validation.pdf.gz | 401.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1al1_full_validation.pdf.gz 1al1_full_validation.pdf.gz | 402.2 KB | Display | |
| Data in XML |  1al1_validation.xml.gz 1al1_validation.xml.gz | 2.4 KB | Display | |
| Data in CIF |  1al1_validation.cif.gz 1al1_validation.cif.gz | 2.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/al/1al1 https://data.pdbj.org/pub/pdb/validation_reports/al/1al1 ftp://data.pdbj.org/pub/pdb/validation_reports/al/1al1 ftp://data.pdbj.org/pub/pdb/validation_reports/al/1al1 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 2 | x 6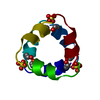
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein/peptide | Mass: 1441.775 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: The peptide was chemically synthesized |
|---|---|
| #2: Chemical | ChemComp-SO4 / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.5 Å3/Da / Density % sol: 64.91 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | *PLUS Temperature: 30 ℃ / pH: 3.24 / Method: vapor diffusion | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.7→10 Å / Rfactor all: 0.255 / Rfactor obs: 0.211 / Data cutoff high absF: 2 | ||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.7→10 Å
| ||||||||||||
| Refine LS restraints |
| ||||||||||||
| Software | *PLUS Name: PROLSQ / Classification: refinement | ||||||||||||
| Refinement | *PLUS σ(F): 2 / Rfactor all: 0.255 / Rfactor obs: 0.211 | ||||||||||||
| Solvent computation | *PLUS | ||||||||||||
| Displacement parameters | *PLUS Biso mean: 25 Å2 |
 Movie
Movie Controller
Controller









 PDBj
PDBj

