[English] 日本語
 Yorodumi
Yorodumi- EMDB-9736: Cryo-EM structure of Murine Norovirus S7 virion with the rising P... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9736 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Murine Norovirus S7 virion with the rising P-domain conformation | |||||||||||||||
 Map data Map data | Murine norovirus S7 virion with the rising P-domain conformation | |||||||||||||||
 Sample Sample |
| |||||||||||||||
| Biological species |  Murine norovirus S7 Murine norovirus S7 | |||||||||||||||
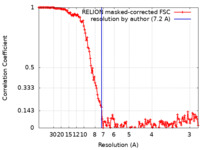
| Method | single particle reconstruction / cryo EM / Resolution: 7.2 Å | |||||||||||||||
 Authors Authors | Song C / Todaka R / Miki M / Haga K / Fujimoto A / Yokoyama M / Miyazaki N / Iwasaki K / Muratami K / Katayama K / Murata K | |||||||||||||||
| Funding support |  Japan, 4 items Japan, 4 items
| |||||||||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2020 Journal: PLoS Pathog / Year: 2020Title: Dynamic rotation of the protruding domain enhances the infectivity of norovirus. Authors: Chihong Song / Reiko Takai-Todaka / Motohiro Miki / Kei Haga / Akira Fujimoto / Ryoka Ishiyama / Kazuki Oikawa / Masaru Yokoyama / Naoyuki Miyazaki / Kenji Iwasaki / Kosuke Murakami / ...Authors: Chihong Song / Reiko Takai-Todaka / Motohiro Miki / Kei Haga / Akira Fujimoto / Ryoka Ishiyama / Kazuki Oikawa / Masaru Yokoyama / Naoyuki Miyazaki / Kenji Iwasaki / Kosuke Murakami / Kazuhiko Katayama / Kazuyoshi Murata /  Abstract: Norovirus is the major cause of epidemic nonbacterial gastroenteritis worldwide. Lack of structural information on infection and replication mechanisms hampers the development of effective vaccines ...Norovirus is the major cause of epidemic nonbacterial gastroenteritis worldwide. Lack of structural information on infection and replication mechanisms hampers the development of effective vaccines and remedies. Here, using cryo-electron microscopy, we show that the capsid structure of murine noroviruses changes in response to aqueous conditions. By twisting the flexible hinge connecting two domains, the protruding (P) domain reversibly rises off the shell (S) domain in solutions of higher pH, but rests on the S domain in solutions of lower pH. Metal ions help to stabilize the resting conformation in this process. Furthermore, in the resting conformation, the cellular receptor CD300lf is readily accessible, and thus infection efficiency is significantly enhanced. Two similar P domain conformations were also found simultaneously in the human norovirus GII.3 capsid, although the mechanism of the conformational change is not yet clear. These results provide new insights into the mechanisms of non-enveloped norovirus transmission that invades host cells, replicates, and sometimes escapes the hosts immune system, through dramatic environmental changes in the gastrointestinal tract. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9736.map.gz emd_9736.map.gz | 55.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9736-v30.xml emd-9736-v30.xml emd-9736.xml emd-9736.xml | 15.6 KB 15.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9736_fsc.xml emd_9736_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_9736.png emd_9736.png | 236.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9736 http://ftp.pdbj.org/pub/emdb/structures/EMD-9736 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9736 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9736 | HTTPS FTP |
-Related structure data
| Related structure data |  9735C  9737C  9738C  9739C  9740C  9741C  6iukC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_9736.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9736.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Murine norovirus S7 virion with the rising P-domain conformation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.422 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Murine norovirus S7
| Entire | Name:  Murine norovirus S7 Murine norovirus S7 |
|---|---|
| Components |
|
-Supramolecule #1: Murine norovirus S7
| Supramolecule | Name: Murine norovirus S7 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1 / NCBI-ID: 357231 / Sci species name: Murine norovirus S7 / Virus type: VIRION / Virus isolate: SPECIES / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  |
| Host system | Organism:  baculovirus / Recombinant cell: RAW264.7 / Recombinant plasmid: cDNA baculovirus / Recombinant cell: RAW264.7 / Recombinant plasmid: cDNA |
| Virus shell | Shell ID: 1 / Diameter: 400.0 Å / T number (triangulation number): 3 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Sugar embedding | Material: amorphous ice |
| Grid | Model: Quantifoil R1.2/1.3 / Material: MOLYBDENUM |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 2200FS |
|---|---|
| Temperature | Min: 76.0 K / Max: 77.0 K |
| Specialist optics | Energy filter - Name: In-column Omega Filter / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: DIRECT ELECTRON DE-20 (5k x 3k) / Digitization - Dimensions - Width: 5120 pixel / Digitization - Dimensions - Height: 3840 pixel / Digitization - Sampling interval: 6.4 µm / Digitization - Frames/image: 3-75 / Number real images: 2739 / Average exposure time: 3.0 sec. / Average electron dose: 15.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 40.0 µm / Calibrated defocus max: 4.9672 µm / Calibrated defocus min: 1.5783 µm / Calibrated magnification: 45065 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 4.2 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 2.0 µm / Nominal magnification: 40000 |
| Sample stage | Specimen holder model: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)