+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9722 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | human LAT1-4F2hc complex bound with BCH | |||||||||||||||||||||
 Map data Map data | cryo EM map | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | transporter / MEMBRANE PROTEIN | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationL-tryptophan transmembrane transport / positive regulation of L-leucine import across plasma membrane / L-tryptophan transmembrane transporter activity / cellular response to L-arginine / apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity ...L-tryptophan transmembrane transport / positive regulation of L-leucine import across plasma membrane / L-tryptophan transmembrane transporter activity / cellular response to L-arginine / apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity / L-alanine import across plasma membrane / Defective SLC7A7 causes lysinuric protein intolerance (LPI) / aromatic amino acid transmembrane transporter activity / phenylalanine transport / methionine transport / L-leucine transmembrane transporter activity / amino acid transmembrane transport / thyroid hormone transmembrane transporter activity / isoleucine transport / valine transport / proline transport / alanine transport / L-amino acid transmembrane transporter activity / L-leucine transport / thyroid hormone transport / negative regulation of vascular associated smooth muscle cell apoptotic process / neutral amino acid transport / positive regulation of cytokine production involved in immune response / amino acid import across plasma membrane / external side of apical plasma membrane / neutral L-amino acid transmembrane transporter activity / Tryptophan catabolism / exogenous protein binding / Amino acid transport across the plasma membrane / amino acid transmembrane transporter activity / anchoring junction / antiporter activity / Basigin interactions / xenobiotic transport / response to muscle activity / microvillus membrane / positive regulation of interleukin-4 production / response to exogenous dsRNA / positive regulation of interleukin-17 production / amino acid transport / tryptophan transport / positive regulation of glial cell proliferation / response to hyperoxia / transport across blood-brain barrier / cellular response to glucose starvation / liver regeneration / negative regulation of autophagy / basal plasma membrane / peptide antigen binding / positive regulation of type II interferon production / calcium ion transport / melanosome / double-stranded RNA binding / cellular response to lipopolysaccharide / virus receptor activity / basolateral plasma membrane / carbohydrate metabolic process / apical plasma membrane / cadherin binding / protein heterodimerization activity / negative regulation of gene expression / lysosomal membrane / intracellular membrane-bounded organelle / synapse / symbiont entry into host cell / cell surface / protein homodimerization activity / RNA binding / extracellular exosome / nucleoplasm / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||||||||||||||
 Authors Authors | Yan RH / Zhao X | |||||||||||||||||||||
| Funding support |  China, 6 items China, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Authors: Renhong Yan / Xin Zhao / Jianlin Lei / Qiang Zhou /  Abstract: The L-type amino acid transporter 1 (LAT1; also known as SLC7A5) catalyses the cross-membrane flux of large neutral amino acids in a sodium- and pH-independent manner. LAT1, an antiporter of the ...The L-type amino acid transporter 1 (LAT1; also known as SLC7A5) catalyses the cross-membrane flux of large neutral amino acids in a sodium- and pH-independent manner. LAT1, an antiporter of the amino acid-polyamine-organocation superfamily, also catalyses the permeation of thyroid hormones, pharmaceutical drugs, and hormone precursors such as L-3,4-dihydroxyphenylalanine across membranes. Overexpression of LAT1 has been observed in a wide range of tumour cells, and it is thus a potential target for anti-cancer drugs. LAT1 forms a heteromeric amino acid transporter complex with 4F2 cell-surface antigen heavy chain (4F2hc; also known as SLC3A2)-a type II membrane glycoprotein that is essential for the stability of LAT1 and for its localization to the plasma membrane. Despite extensive cell-based characterization of the LAT1-4F2hc complex and structural determination of its homologues in bacteria, the interactions between LAT1 and 4F2hc and the working mechanism of the complex remain largely unknown. Here we report the cryo-electron microscopy structures of human LAT1-4F2hc alone and in complex with the inhibitor 2-amino-2-norbornanecarboxylic acid at resolutions of 3.3 Å and 3.5 Å, respectively. LAT1 exhibits an inward open conformation. Besides a disulfide bond association, LAT1 also interacts extensively with 4F2hc on the extracellular side, within the membrane, and on the intracellular side. Biochemical analysis reveals that 4F2hc is essential for the transport activity of the complex. Together, our characterizations shed light on the architecture of the LAT1-4F2hc complex, and provide insights into its function and the mechanisms through which it might be associated with disease. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9722.map.gz emd_9722.map.gz | 59.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9722-v30.xml emd-9722-v30.xml emd-9722.xml emd-9722.xml | 19.8 KB 19.8 KB | Display Display |  EMDB header EMDB header |
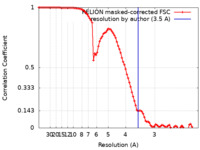
| FSC (resolution estimation) |  emd_9722_fsc.xml emd_9722_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_9722.png emd_9722.png | 52.1 KB | ||
| Filedesc metadata |  emd-9722.cif.gz emd-9722.cif.gz | 7.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9722 http://ftp.pdbj.org/pub/emdb/structures/EMD-9722 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9722 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9722 | HTTPS FTP |
-Validation report
| Summary document |  emd_9722_validation.pdf.gz emd_9722_validation.pdf.gz | 518.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_9722_full_validation.pdf.gz emd_9722_full_validation.pdf.gz | 518 KB | Display | |
| Data in XML |  emd_9722_validation.xml.gz emd_9722_validation.xml.gz | 10.8 KB | Display | |
| Data in CIF |  emd_9722_validation.cif.gz emd_9722_validation.cif.gz | 14.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9722 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9722 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9722 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-9722 | HTTPS FTP |
-Related structure data
| Related structure data |  6irtMC  0678C  0679C  9721C  6irsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9722.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9722.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo EM map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.091 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human LAT1-4F2hc complex bound with BCH
| Entire | Name: human LAT1-4F2hc complex bound with BCH |
|---|---|
| Components |
|
-Supramolecule #1: human LAT1-4F2hc complex bound with BCH
| Supramolecule | Name: human LAT1-4F2hc complex bound with BCH / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 100 KDa |
-Macromolecule #1: 4F2 cell-surface antigen heavy chain
| Macromolecule | Name: 4F2 cell-surface antigen heavy chain / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 70.160828 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAHHHHHHHH HHSGRELQPP EASIAVVSIP RQLPGSHSEA GVQGLSAGDD SETGSDCVTQ AGLQLLASSD PPALASKNAE VTVETGFHH VSQADIEFLT SIDPTASASG SAGITGTMSQ DTEVDMKEVE LNELEPEKQP MNAASGAAMS LAGAEKNGLV K IKVAEDEA ...String: MAHHHHHHHH HHSGRELQPP EASIAVVSIP RQLPGSHSEA GVQGLSAGDD SETGSDCVTQ AGLQLLASSD PPALASKNAE VTVETGFHH VSQADIEFLT SIDPTASASG SAGITGTMSQ DTEVDMKEVE LNELEPEKQP MNAASGAAMS LAGAEKNGLV K IKVAEDEA EAAAAAKFTG LSKEELLKVA GSPGWVRTRW ALLLLFWLGW LGMLAGAVVI IVRAPRCREL PAQKWWHTGA LY RIGDLQA FQGHGAGNLA GLKGRLDYLS SLKVKGLVLG PIHKNQKDDV AQTDLLQIDP NFGSKEDFDS LLQSAKKKSI RVI LDLTPN YRGENSWFST QVDTVATKVK DALEFWLQAG VDGFQVRDIE NLKDASSFLA EWQNITKGFS EDRLLIAGTN SSDL QQILS LLESNKDLLL TSSYLSDSGS TGEHTKSLVT QYLNATGNRW CSWSLSQARL LTSFLPAQLL RLYQLMLFTL PGTPV FSYG DEIGLDAAAL PGQPMEAPVM LWDESSFPDI PGAVSANMTV KGQSEDPGSL LSLFRRLSDQ RSKERSLLHG DFHAFS AGP GLFSYIRHWD QNERFLVVLN FGDVGLSAGL QASDLPASAS LPAKADLLLS TQPGREEGSP LELERLKLEP HEGLLLR FP YAALE UniProtKB: Amino acid transporter heavy chain SLC3A2 |
-Macromolecule #2: Large neutral amino acids transporter small subunit 1
| Macromolecule | Name: Large neutral amino acids transporter small subunit 1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 57.244934 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MADYKDDDDK SGPDEVDASG RAGAGPKRRA LAAPAAEEKE EAREKMLAAK SADGSEPAGE GEGVTLQRNI TLLNGVAIIV GTIIGSGIF VTPTGVLKEA GSPGLALVVW AACGVFSIVG ALCYAELGTT ISKSGGDYAY MLEVYGSLPA FLKLWIELLI I RPSSQYIV ...String: MADYKDDDDK SGPDEVDASG RAGAGPKRRA LAAPAAEEKE EAREKMLAAK SADGSEPAGE GEGVTLQRNI TLLNGVAIIV GTIIGSGIF VTPTGVLKEA GSPGLALVVW AACGVFSIVG ALCYAELGTT ISKSGGDYAY MLEVYGSLPA FLKLWIELLI I RPSSQYIV ALVFATYLLK PLFPTCPVPE EAAKLVACLC VLLLTAVNCY SVKAATRVQD AFAAAKLLAL ALIILLGFVQ IG KGDVSNL DPNFSFEGTK LDVGNIVLAL YSGLFAYGGW NYLNFVTEEM INPYRNLPLA IIISLPIVTL VYVLTNLAYF TTL STEQML SSEAVAVDFG NYHLGVMSWI IPVFVGLSCF GSVNGSLFTS SRLFFVGSRE GHLPSILSMI HPQLLTPVPS LVFT CVMTL LYAFSKDIFS VINFFSFFNW LCVALAIIGM IWLRHRKPEL ERPIKVNLAL PVFFILACLF LIAVSFWKTP VECGI GFTI ILSGLPVYFF GVWWKNKPKW LLQGIFSTTV LCQKLMQVVP QET UniProtKB: Large neutral amino acids transporter small subunit 1 |
-Macromolecule #4: 1,2-DIACYL-GLYCEROL-3-SN-PHOSPHATE
| Macromolecule | Name: 1,2-DIACYL-GLYCEROL-3-SN-PHOSPHATE / type: ligand / ID: 4 / Number of copies: 2 / Formula: 3PH |
|---|---|
| Molecular weight | Theoretical: 704.998 Da |
| Chemical component information |  ChemComp-3PH: |
-Macromolecule #5: (1S,2S,4R)-2-aminobicyclo[2.2.1]heptane-2-carboxylic acid
| Macromolecule | Name: (1S,2S,4R)-2-aminobicyclo[2.2.1]heptane-2-carboxylic acid type: ligand / ID: 5 / Number of copies: 1 / Formula: AUU |
|---|---|
| Molecular weight | Theoretical: 155.194 Da |
| Chemical component information |  ChemComp-AUU: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)