+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8685 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | GluA2-0xGSG1L bound to ZK | ||||||||||||||||||||||||
 Map data Map data | GluA2-0xGSG1L bound to ZK | ||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
 Keywords Keywords | Ion channel / TRANSPORT PROTEIN | ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of AMPA receptor activity / regulation of short-term neuronal synaptic plasticity / spine synapse / dendritic spine cytoplasm / dendritic spine neck / cellular response to amine stimulus / dendritic spine head / regulation of postsynaptic neurotransmitter receptor internalization / Activation of AMPA receptors / ligand-gated monoatomic cation channel activity ...regulation of AMPA receptor activity / regulation of short-term neuronal synaptic plasticity / spine synapse / dendritic spine cytoplasm / dendritic spine neck / cellular response to amine stimulus / dendritic spine head / regulation of postsynaptic neurotransmitter receptor internalization / Activation of AMPA receptors / ligand-gated monoatomic cation channel activity / perisynaptic space / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors / response to lithium ion / transmission of nerve impulse / AMPA glutamate receptor clustering / cellular response to glycine / kainate selective glutamate receptor activity / immunoglobulin binding / asymmetric synapse / AMPA glutamate receptor complex / regulation of receptor recycling / extracellularly glutamate-gated ion channel activity / ionotropic glutamate receptor complex / conditioned place preference / Unblocking of NMDA receptors, glutamate binding and activation / glutamate receptor binding / positive regulation of synaptic transmission / regulation of synaptic transmission, glutamatergic / response to fungicide / cytoskeletal protein binding / extracellular ligand-gated monoatomic ion channel activity / glutamate-gated receptor activity / cellular response to brain-derived neurotrophic factor stimulus / regulation of long-term synaptic depression / somatodendritic compartment / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / dendrite membrane / ionotropic glutamate receptor binding / excitatory synapse / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / synaptic membrane / positive regulation of excitatory postsynaptic potential / dendritic shaft / SNARE binding / PDZ domain binding / protein tetramerization / synaptic transmission, glutamatergic / establishment of protein localization / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / synapse organization / cerebral cortex development / receptor internalization / postsynaptic density membrane / modulation of chemical synaptic transmission / Schaffer collateral - CA1 synapse / terminal bouton / synaptic vesicle / long-term synaptic potentiation / synaptic vesicle membrane / signaling receptor activity / amyloid-beta binding / presynapse / growth cone / presynaptic membrane / scaffold protein binding / dendritic spine / chemical synaptic transmission / perikaryon / postsynaptic membrane / neuron projection / postsynaptic density / external side of plasma membrane / axon / neuronal cell body / dendrite / synapse / protein kinase binding / protein-containing complex binding / glutamatergic synapse / cell surface / endoplasmic reticulum / protein-containing complex / identical protein binding / membrane / plasma membrane Similarity search - Function | ||||||||||||||||||||||||
| Biological species |  | ||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 7.8 Å | ||||||||||||||||||||||||
 Authors Authors | Twomey EC / Yelshanskaya MV | ||||||||||||||||||||||||
| Funding support |  United States, 7 items United States, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Neuron / Year: 2017 Journal: Neuron / Year: 2017Title: Structural Bases of Desensitization in AMPA Receptor-Auxiliary Subunit Complexes. Authors: Edward C Twomey / Maria V Yelshanskaya / Robert A Grassucci / Joachim Frank / Alexander I Sobolevsky /  Abstract: Fast excitatory neurotransmission is mediated by AMPA-subtype ionotropic glutamate receptors (AMPARs). AMPARs, localized at post-synaptic densities, are regulated by transmembrane auxiliary subunits ...Fast excitatory neurotransmission is mediated by AMPA-subtype ionotropic glutamate receptors (AMPARs). AMPARs, localized at post-synaptic densities, are regulated by transmembrane auxiliary subunits that modulate AMPAR assembly, trafficking, gating, and pharmacology. Aberrancies in AMPAR-mediated signaling are associated with numerous neurological disorders. Here, we report cryo-EM structures of an AMPAR in complex with the auxiliary subunit GSG1L in the closed and desensitized states. GSG1L favors the AMPAR desensitized state, where channel closure is facilitated by profound structural rearrangements in the AMPAR extracellular domain, with ligand-binding domain dimers losing their local 2-fold rotational symmetry. Our structural and functional experiments suggest that AMPAR auxiliary subunits share a modular architecture and use a common transmembrane scaffold for distinct extracellular modules to differentially regulate AMPAR gating. By comparing the AMPAR-GSG1L complex structures, we map conformational changes accompanying AMPAR recovery from desensitization and reveal structural bases for regulation of synaptic transmission by auxiliary subunits. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8685.map.gz emd_8685.map.gz | 11.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8685-v30.xml emd-8685-v30.xml emd-8685.xml emd-8685.xml | 13.1 KB 13.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8685.png emd_8685.png | 30.7 KB | ||
| Filedesc metadata |  emd-8685.cif.gz emd-8685.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8685 http://ftp.pdbj.org/pub/emdb/structures/EMD-8685 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8685 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8685 | HTTPS FTP |
-Related structure data
| Related structure data |  5vhwMC  8686C  8687C  8688C  5vhxC  5vhyC  5vhzC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8685.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8685.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | GluA2-0xGSG1L bound to ZK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
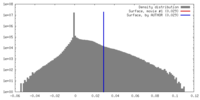
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : GluA2-0xGSG1L
| Entire | Name: GluA2-0xGSG1L |
|---|---|
| Components |
|
-Supramolecule #1: GluA2-0xGSG1L
| Supramolecule | Name: GluA2-0xGSG1L / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Glutamate receptor 2,Germ cell-specific gene 1-like protein
| Macromolecule | Name: Glutamate receptor 2,Germ cell-specific gene 1-like protein type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 117.471211 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NSIQIGGLFP RGADQEYSAF RVGMVQFSTS EFRLTPHIDN LEVANSFAVT NAFCSQFSRG VYAIFGFYDK KSVNTITSFC GTLHVSFIT PSFPTDGTHP FVIQMRPDLK GALLSLIEYY QWDKFAYLYD SDRGLSTLQA VLDSAAEKKW QVTAINVGNI N NDKKDETY ...String: NSIQIGGLFP RGADQEYSAF RVGMVQFSTS EFRLTPHIDN LEVANSFAVT NAFCSQFSRG VYAIFGFYDK KSVNTITSFC GTLHVSFIT PSFPTDGTHP FVIQMRPDLK GALLSLIEYY QWDKFAYLYD SDRGLSTLQA VLDSAAEKKW QVTAINVGNI N NDKKDETY RSLFQDLELK KERRVILDCE RDKVNDIVDQ VITIGKHVKG YHYIIANLGF TDGDLLKIQF GGAEVSGFQI VD YDDSLVS KFIERWSTLE EKEYPGAHTA TIKYTSALTY DAVQVMTEAF RNLRKQRIEI SRRGNAGDCL ANPAVPWGQG VEI ERALKQ VQVEGLSGNI KFDQNGKRIN YTINIMELKT NGPRKIGYWS EVDKMVLTED DTSGLEQKTV VVTTILESPY VMMK KNHEM LEGNERYEGY CVDLAAEIAK HCGFKYKLTI VGDGKYGARD ADTKIWNGMV GELVYGKADI AIAPLTITLV REEVI DFSK PFMSLGISIM IKKPQKSKPG VFSFLDPLAY EIWMCIVFAY IGVSVVLFLV SRFSPYEWHT EEFEDGRETQ SSESTN EFG IFNSLWFSLG AFMQQGCDIS PRSLSGRIVG GVWWFFTLII ISSYTANLAA FLTVERMVSP IESAEDLSKQ TEIAYGT LD SGSTKEFFRR SKIAVFDKMW TYMRSAEPSV FVRTTAEGVA RVRKSKGKYA YLLESTMNEY IEQRKPCDTM KVGGNLDS K GYGIATPKGS SLGTPVNLAV LKLSEQGVLD KLKNKWWYDK GECGAKDSGS KEKTSALSLS NVAGVFYILV GGLGLAMLV ALIEFCYKSR AEAKRMKGTG KTSRRGRALL AVALNLLALL FATTAFLTTY WCQGTQRVPK PGCGQGGGAN CPNSGANATA NSTAAPVAA SPAGAPYSWE AGDERFQLRR FHTGIWYSCE EELGGPGEKC RSFIDLAPAS EKGVLWLSVV SEVLYILLLV V GFSLMCLE LLHSSSVIDG LKLNAFAAVF TVLSGLLGMV AHMMYTQVFQ VTVSLGPEDW RPHSWDYGWS FCLAWGSFTC CM AASVTTL NSYTKTVIEF UniProtKB: Glutamate receptor 2, Germ cell-specific gene 1-like protein |
-Macromolecule #2: {[7-morpholin-4-yl-2,3-dioxo-6-(trifluoromethyl)-3,4-dihydroquino...
| Macromolecule | Name: {[7-morpholin-4-yl-2,3-dioxo-6-(trifluoromethyl)-3,4-dihydroquinoxalin-1(2H)-yl]methyl}phosphonic acid type: ligand / ID: 2 / Number of copies: 4 / Formula: ZK1 |
|---|---|
| Molecular weight | Theoretical: 409.254 Da |
| Chemical component information | 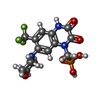 ChemComp-ZK1: |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 4 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: C-flat-1.2/1.3 / Material: GOLD / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE Details: Gold-gold grids, hydrogen and oxygen glow discharge (20s, 10 watts, 6.4 sccm H2, 27.5 sccm O2) |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C2 (2 fold cyclic) / Algorithm: FOURIER SPACE / Resolution.type: BY AUTHOR / Resolution: 7.8 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 14372 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)