+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8542 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Phosphofructokinase-1 Filamnet | ||||||||||||
 Map data Map data | wildtype phosphofructokinase liver isoform filaments assembled with ATP and fructose-6-phosphate | ||||||||||||
 Sample Sample |
| ||||||||||||
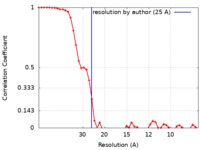
| Method | helical reconstruction / negative staining / Resolution: 25.0 Å | ||||||||||||
 Authors Authors | Dosey AM / Kollman JM | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: J Cell Biol / Year: 2017 Journal: J Cell Biol / Year: 2017Title: The glycolytic enzyme phosphofructokinase-1 assembles into filaments. Authors: Bradley A Webb / Anne M Dosey / Torsten Wittmann / Justin M Kollman / Diane L Barber /  Abstract: Despite abundant knowledge of the regulation and biochemistry of glycolytic enzymes, we have limited understanding on how they are spatially organized in the cell. Emerging evidence indicates that ...Despite abundant knowledge of the regulation and biochemistry of glycolytic enzymes, we have limited understanding on how they are spatially organized in the cell. Emerging evidence indicates that nonglycolytic metabolic enzymes regulating diverse pathways can assemble into polymers. We now show tetramer- and substrate-dependent filament assembly by phosphofructokinase-1 (PFK1), which is considered the "gatekeeper" of glycolysis because it catalyzes the step committing glucose to breakdown. Recombinant liver PFK1 (PFKL) isoform, but not platelet PFK1 (PFKP) or muscle PFK1 (PFKM) isoforms, assembles into filaments. Negative-stain electron micrographs reveal that filaments are apolar and made of stacked tetramers oriented with exposed catalytic sites positioned along the edge of the polymer. Electron micrographs and biochemical data with a PFKL/PFKP chimera indicate that the PFKL regulatory domain mediates filament assembly. Quantified live-cell imaging shows dynamic properties of localized PFKL puncta that are enriched at the plasma membrane. These findings reveal a new behavior of a key glycolytic enzyme with insights on spatial organization and isoform-specific glucose metabolism in cells. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8542.map.gz emd_8542.map.gz | 6.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8542-v30.xml emd-8542-v30.xml emd-8542.xml emd-8542.xml | 11.1 KB 11.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8542_fsc.xml emd_8542_fsc.xml | 5.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_8542.png emd_8542.png | 68.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8542 http://ftp.pdbj.org/pub/emdb/structures/EMD-8542 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8542 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8542 | HTTPS FTP |
-Validation report
| Summary document |  emd_8542_validation.pdf.gz emd_8542_validation.pdf.gz | 79.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8542_full_validation.pdf.gz emd_8542_full_validation.pdf.gz | 78.2 KB | Display | |
| Data in XML |  emd_8542_validation.xml.gz emd_8542_validation.xml.gz | 494 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8542 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8542 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8542 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8542 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_8542.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8542.map.gz / Format: CCP4 / Size: 6.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | wildtype phosphofructokinase liver isoform filaments assembled with ATP and fructose-6-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.14 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Filament of phosphofructokinase-1 liver isoform
| Entire | Name: Filament of phosphofructokinase-1 liver isoform |
|---|---|
| Components |
|
-Supramolecule #1: Filament of phosphofructokinase-1 liver isoform
| Supramolecule | Name: Filament of phosphofructokinase-1 liver isoform / type: complex / ID: 1 / Parent: 0 Details: PFKL tetramers assembled into filaments by addition of ATP and fructose-6-phosphate |
|---|---|
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 42.2 kDa/nm |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Staining | Type: NEGATIVE / Material: uranyl formate |
| Grid | Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI SPIRIT |
|---|---|
| Image recording | Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 120 / Average exposure time: 1.2 sec. / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
| Experimental equipment |  Model: Tecnai Spirit / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



 UCSF Chimera
UCSF Chimera



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)