+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-8404 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Single particle helical reconstruction of protease cleavage product from HIV-1 Gag VLPs | ||||||||||||
 マップデータ マップデータ | CA tube assembled from gag cleavage | ||||||||||||
 試料 試料 |
| ||||||||||||
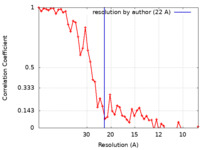
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / 解像度: 22.0 Å | ||||||||||||
 データ登録者 データ登録者 | Fu X / Zhang P | ||||||||||||
| 資金援助 |  米国, 3件 米国, 3件
| ||||||||||||
 引用 引用 |  ジャーナル: Nat Commun / 年: 2016 ジャーナル: Nat Commun / 年: 2016タイトル: In vitro protease cleavage and computer simulations reveal the HIV-1 capsid maturation pathway. 著者: Jiying Ning / Gonca Erdemci-Tandogan / Ernest L Yufenyuy / Jef Wagner / Benjamin A Himes / Gongpu Zhao / Christopher Aiken / Roya Zandi / Peijun Zhang /   要旨: HIV-1 virions assemble as immature particles containing Gag polyproteins that are processed by the viral protease into individual components, resulting in the formation of mature infectious particles. ...HIV-1 virions assemble as immature particles containing Gag polyproteins that are processed by the viral protease into individual components, resulting in the formation of mature infectious particles. There are two competing models for the process of forming the mature HIV-1 core: the disassembly and de novo reassembly model and the non-diffusional displacive model. To study the maturation pathway, we simulate HIV-1 maturation in vitro by digesting immature particles and assembled virus-like particles with recombinant HIV-1 protease and monitor the process with biochemical assays and cryoEM structural analysis in parallel. Processing of Gag in vitro is accurate and efficient and results in both soluble capsid protein and conical or tubular capsid assemblies, seemingly converted from immature Gag particles. Computer simulations further reveal probable assembly pathways of HIV-1 capsid formation. Combining the experimental data and computer simulations, our results suggest a sequential combination of both displacive and disassembly/reassembly processes for HIV-1 maturation. | ||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_8404.map.gz emd_8404.map.gz | 2.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-8404-v30.xml emd-8404-v30.xml emd-8404.xml emd-8404.xml | 12.7 KB 12.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_8404_fsc.xml emd_8404_fsc.xml | 5.5 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_8404.png emd_8404.png | 203.1 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8404 http://ftp.pdbj.org/pub/emdb/structures/EMD-8404 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8404 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8404 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_8404_validation.pdf.gz emd_8404_validation.pdf.gz | 386.5 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_8404_full_validation.pdf.gz emd_8404_full_validation.pdf.gz | 386.1 KB | 表示 | |
| XML形式データ |  emd_8404_validation.xml.gz emd_8404_validation.xml.gz | 8.5 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8404 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8404 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8404 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8404 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_8404.map.gz / 形式: CCP4 / 大きさ: 8.4 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_8404.map.gz / 形式: CCP4 / 大きさ: 8.4 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | CA tube assembled from gag cleavage | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 4.52 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Single particle helical reconstruction of protease cleavage produ...
| 全体 | 名称: Single particle helical reconstruction of protease cleavage product from HIV-1 Gag VLPs |
|---|---|
| 要素 |
|
-超分子 #1: Single particle helical reconstruction of protease cleavage produ...
| 超分子 | 名称: Single particle helical reconstruction of protease cleavage product from HIV-1 Gag VLPs タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1 |
|---|
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | helical array |
- 試料調製
試料調製
| 濃度 | 2.0 mg/mL |
|---|---|
| 緩衝液 | pH: 6 / 構成要素 - 濃度: 50.0 mM / 構成要素 - 式: NaAc / 構成要素 - 名称: Sodium Acetate |
| グリッド | モデル: Quantifoil R2/1 / 材質: COPPER / メッシュ: 400 / 支持フィルム - 材質: CARBON / 支持フィルム - トポロジー: HOLEY / 前処理 - タイプ: GLOW DISCHARGE |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 298 K / 装置: HOMEMADE PLUNGER |
| 詳細 | sample is resulted from HIV-1 protease cleavage of in vitro assembled Gag VLPs |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TECNAI F20 |
|---|---|
| 特殊光学系 | 球面収差補正装置: No / 色収差補正装置: No / エネルギーフィルター - 名称: No |
| 撮影 | フィルム・検出器のモデル: GATAN ULTRASCAN 4000 (4k x 4k) デジタル化 - サイズ - 横: 4096 pixel / デジタル化 - サイズ - 縦: 4096 pixel / デジタル化 - サンプリング間隔: 15.0 µm / 撮影したグリッド数: 1 / 実像数: 2 / 平均露光時間: 1.0 sec. / 平均電子線量: 10.0 e/Å2 詳細: Image was collected by single exposure, not movie mode. |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | C2レンズ絞り径: 100.0 µm / 最大 デフォーカス(補正後): 9.0 µm / 最小 デフォーカス(補正後): 8.0 µm / 倍率(補正後): 66262 / 照射モード: OTHER / 撮影モード: BRIGHT FIELD / Cs: 2.0 mm / 倍率(公称値): 50000 |
| 試料ステージ | 試料ホルダーモデル: GATAN 626 SINGLE TILT LIQUID NITROGEN CRYO TRANSFER HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Tecnai F20 / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)