+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8284 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | expanded poliovirus in complex with VHH 12B | |||||||||
 Map data Map data | expanded poliovirus in complex with VHH 12B | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | poliovirus / VHH / nanobody / 80S / expanded / single domain antibody / VIRUS-immune system complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host translation initiation / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane ...symbiont-mediated suppression of host translation initiation / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |   Poliovirus type 1 (strain Mahoney) / Poliovirus type 1 (strain Mahoney) /  Poliovirus type 1 / Poliovirus type 1 /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.3 Å | |||||||||
 Authors Authors | Strauss M / Schotte L / Filman DJ / Hogle JM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: J Virol / Year: 2017 Journal: J Virol / Year: 2017Title: Cryo-electron Microscopy Structures of Expanded Poliovirus with VHHs Sample the Conformational Repertoire of the Expanded State. Authors: Mike Strauss / Lise Schotte / Krishanthi S Karunatilaka / David J Filman / James M Hogle /   Abstract: By using cryo-electron microscopy, expanded 80S-like poliovirus virions (poliovirions) were visualized in complexes with four 80S-specific camelid VHHs (Nanobodies). In all four complexes, the VHHs ...By using cryo-electron microscopy, expanded 80S-like poliovirus virions (poliovirions) were visualized in complexes with four 80S-specific camelid VHHs (Nanobodies). In all four complexes, the VHHs bind to a site on the top surface of the capsid protein VP3, which is hidden in the native virus. Interestingly, although the four VHHs bind to the same site, the structures of the expanded virus differ in detail in each complex, suggesting that each of the Nanobodies has sampled a range of low-energy structures available to the expanded virion. By stabilizing unique structures of expanded virions, VHH binding permitted a more detailed view of the virus structure than was previously possible, leading to a better understanding of the expansion process that is a critical step in infection. It is now clear which polypeptide chains become disordered and which become rearranged. The higher resolution of these structures also revealed well-ordered conformations for the EF loop of VP2, the GH loop of VP3, and the N-terminal extensions of VP1 and VP2, which, in retrospect, were present in lower-resolution structures but not recognized. These structural observations help to explain preexisting mutational data and provide insights into several other stages of the poliovirus life cycle, including the mechanism of receptor-triggered virus expansion. IMPORTANCE: When poliovirus infects a cell, it undergoes a change in its structure in order to pass RNA through its protein coat, but this altered state is short-lived and thus poorly understood. The ...IMPORTANCE: When poliovirus infects a cell, it undergoes a change in its structure in order to pass RNA through its protein coat, but this altered state is short-lived and thus poorly understood. The structures of poliovirus bound to single-domain antibodies presented here capture the altered virus in what appear to be intermediate states. A careful analysis of these structures lets us better understand the molecular mechanism of infection and how these changes in the virus lead to productive-infection events. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8284.map.gz emd_8284.map.gz | 387.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8284-v30.xml emd-8284-v30.xml emd-8284.xml emd-8284.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8284.png emd_8284.png | 187.3 KB | ||
| Filedesc metadata |  emd-8284.cif.gz emd-8284.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8284 http://ftp.pdbj.org/pub/emdb/structures/EMD-8284 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8284 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8284 | HTTPS FTP |
-Related structure data
| Related structure data |  5ktzMC  8277C  8285C  8286C  8292C  5ku0C  5ku2C  5kwlC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8284.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8284.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | expanded poliovirus in complex with VHH 12B | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.986 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
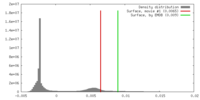
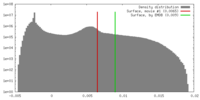
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : expanded poliovirus in complex with VHH 12B
| Entire | Name: expanded poliovirus in complex with VHH 12B |
|---|---|
| Components |
|
-Supramolecule #1: expanded poliovirus in complex with VHH 12B
| Supramolecule | Name: expanded poliovirus in complex with VHH 12B / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Poliovirus type 1 (strain Mahoney) Poliovirus type 1 (strain Mahoney) |
| Molecular weight | Theoretical: 9 MDa |
-Macromolecule #1: VP1
| Macromolecule | Name: VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Poliovirus type 1 (strain Mahoney) / Strain: Mahoney Poliovirus type 1 (strain Mahoney) / Strain: Mahoney |
| Molecular weight | Theoretical: 25.291594 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: PSDTVQTRHV VQHRSRSESS IESFFARGAC VTIMTVDNPA STTNKDKLFA VWKITYKDTV QLRRKLEFFT YSRFDMELTF VVTANFTET NNGHALNQVY QIMYVPPGAP VPEKWDDYTW QTSSNPSIFY TYGTAPARIS VPYVGISNAY SHFYDGFSKV P LKDQSAAL ...String: PSDTVQTRHV VQHRSRSESS IESFFARGAC VTIMTVDNPA STTNKDKLFA VWKITYKDTV QLRRKLEFFT YSRFDMELTF VVTANFTET NNGHALNQVY QIMYVPPGAP VPEKWDDYTW QTSSNPSIFY TYGTAPARIS VPYVGISNAY SHFYDGFSKV P LKDQSAAL GDSIYGAASL NDFGILAVRV VNDHNPTKVT SKIRVYLKPK HIRVWCPRPP RAVAY UniProtKB: Genome polyprotein |
-Macromolecule #2: VP2
| Macromolecule | Name: VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Poliovirus type 1 (strain Mahoney) / Strain: Mahoney Poliovirus type 1 (strain Mahoney) / Strain: Mahoney |
| Molecular weight | Theoretical: 29.677301 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: SPNIEACGYS DRVLQLTLGN STITTQEAAN SVVAYGRWPE YLRDSEANPV DQPTEPDVAA CRFYTLDTVS WTKESRGWWW KLPDALRDM GLFGQNMYYH YLGRSGYTVH VQCNASKFHQ GALGVFAVPE MCLAGDSNTT TMHTSYQNAN PGEKGGTFTG T FTPDNNQT ...String: SPNIEACGYS DRVLQLTLGN STITTQEAAN SVVAYGRWPE YLRDSEANPV DQPTEPDVAA CRFYTLDTVS WTKESRGWWW KLPDALRDM GLFGQNMYYH YLGRSGYTVH VQCNASKFHQ GALGVFAVPE MCLAGDSNTT TMHTSYQNAN PGEKGGTFTG T FTPDNNQT SPARRFCPVD YLLGNGTLLG NAFVFPHQII NLRTNNCATL VLPYVNSLSI DSMVKHNNWG IAILPLAPLN FA SESSPEI PITLTIAPMC CEFNGLRNIT LP UniProtKB: Genome polyprotein |
-Macromolecule #3: Genome polyprotein
| Macromolecule | Name: Genome polyprotein / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Poliovirus type 1 / Strain: Mahoney Poliovirus type 1 / Strain: Mahoney |
| Molecular weight | Theoretical: 25.777613 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GLPVMNTPGS NQYLTADNFQ SPCALPEFDV TPPIDIPGEV KNMMELAEID TMIPFDLSAT KKNTMEMYRV RLSDKPHTDD PILCLSLSP ASDPRLSHTM LGEILNYYTH WAGSLKFTFL FCGSMMATGK LLVSYAPPGA DPPKKRKEAM LGTHVIWDIG L QSSCTMVV ...String: GLPVMNTPGS NQYLTADNFQ SPCALPEFDV TPPIDIPGEV KNMMELAEID TMIPFDLSAT KKNTMEMYRV RLSDKPHTDD PILCLSLSP ASDPRLSHTM LGEILNYYTH WAGSLKFTFL FCGSMMATGK LLVSYAPPGA DPPKKRKEAM LGTHVIWDIG L QSSCTMVV PWISNTTYRQ TIDDSFTEGG YISVFYQTRI VVPLSTPREM DILGFVSACN DFSVRLLRDT THI UniProtKB: Genome polyprotein |
-Macromolecule #4: VHH 12B
| Macromolecule | Name: VHH 12B / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.072334 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG SVQAGGSLTL SCAASGYAVS RYSMGWFRQA PGKENEGVAA IDSSGVGTTY ADSVKGRFTI SRDNAKDTVY LRMNSLKPE DTAIYYCASG FGLSLSRYTY AYWGQGTQVT VSSH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.45 mg/mL |
|---|---|
| Buffer | pH: 7 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: Details: A map was generated from the PDB and low-pass filtered to 6 nm. |
|---|---|
| Final reconstruction | Applied symmetry - Point group: I (icosahedral) / Resolution.type: BY AUTHOR / Resolution: 4.3 Å / Resolution method: FSC 0.143 CUT-OFF / Software: (Name: GeFrealign, FREALIGN (ver. 9.09)) / Number images used: 17627 |
| Initial angle assignment | Type: PROJECTION MATCHING / Software - Name: RELION (ver. 1.3) |
| Final angle assignment | Type: PROJECTION MATCHING / Software - Name: FREALIGN (ver. 9) |
-Atomic model buiding 1
| Details | Fitting protocol: rigid body restraint of structurally conserved areas, and stereochemically restrained, icosahedrally restrained flexible fitting of areas that would otherwise disagree with the map. Refinement space: reciprocal, using both Fourier amplitudes and phases. |
|---|---|
| Refinement | Space: RECIPROCAL / Protocol: OTHER |
| Output model |  PDB-5ktz: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)