[English] 日本語
 Yorodumi
Yorodumi- EMDB-8126: Dimeric N-BAR domain of human Bin1 (Amphiphysin2) associated with... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8126 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Dimeric N-BAR domain of human Bin1 (Amphiphysin2) associated with tubular sarcolemma model membrane | |||||||||
 Map data Map data | None | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 50.0 Å | |||||||||
 Authors Authors | Daum B / Meister A / Kuehlbrandt W | |||||||||
 Citation Citation |  Journal: J Struct Biol / Year: 2016 Journal: J Struct Biol / Year: 2016Title: Supramolecular organization of the human N-BAR domain in shaping the sarcolemma membrane. Authors: Bertram Daum / Andrea Auerswald / Tobias Gruber / Gerd Hause / Jochen Balbach / Werner Kühlbrandt / Annette Meister /  Abstract: The 30kDa N-BAR domain of the human Bin1 protein is essential for the generation of skeletal muscle T-tubules. By electron cryo-microscopy and electron cryo-tomography with a direct electron ...The 30kDa N-BAR domain of the human Bin1 protein is essential for the generation of skeletal muscle T-tubules. By electron cryo-microscopy and electron cryo-tomography with a direct electron detector, we found that Bin1-N-BAR domains assemble into scaffolds of low long-range order that form flexible membrane tubules. The diameter of the tubules closely matches the curved shape of the N-BAR domain, which depends on the composition of the target membrane. These insights are fundamental to our understanding of T-tubule formation and function in human skeletal muscle. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8126.map.gz emd_8126.map.gz | 2.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8126-v30.xml emd-8126-v30.xml emd-8126.xml emd-8126.xml | 10.2 KB 10.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8126.png emd_8126.png | 150.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8126 http://ftp.pdbj.org/pub/emdb/structures/EMD-8126 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8126 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8126 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_8126.map.gz / Format: CCP4 / Size: 3.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8126.map.gz / Format: CCP4 / Size: 3.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 7.7066 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Dimeric N-BAR domain of human Bin1 (Amphiphysin2) associated with...
| Entire | Name: Dimeric N-BAR domain of human Bin1 (Amphiphysin2) associated with sarcolemma model membrane tube |
|---|---|
| Components |
|
-Supramolecule #1: Dimeric N-BAR domain of human Bin1 (Amphiphysin2) associated with...
| Supramolecule | Name: Dimeric N-BAR domain of human Bin1 (Amphiphysin2) associated with sarcolemma model membrane tube type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 60 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 3200FSC |
|---|---|
| Specialist optics | Energy filter - Name: In-column Omega Filter |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 0.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 3.5 µm / Calibrated defocus min: 2.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 4.1 mm / Nominal magnification: 10000 |
| Sample stage | Specimen holder model: JEOL 3200FSC CRYOHOLDER / Cooling holder cryogen: NITROGEN |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Point group: C2 (2 fold cyclic) / Resolution.type: BY AUTHOR / Resolution: 50.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software: (Name: PEET,  IMOD) / Number subtomograms used: 446 IMOD) / Number subtomograms used: 446 |
|---|---|
| Extraction | Number tomograms: 1 / Number images used: 446 / Software - Name:  IMOD IMOD |
| CTF correction | Software - Name:  IMOD IMOD |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller



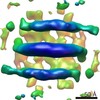

 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















