[English] 日本語
 Yorodumi
Yorodumi- EMDB-7816: Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7816 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with GABA and flumazenil, conformation B | |||||||||
 Map data Map data | Main map sharpened to B factor -153 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ligand-gated ion channel / GABA-A receptor / Cys-loop receptor / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationbenzodiazepine receptor activity / GABA receptor complex / cellular response to histamine / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-gated chloride ion channel activity / GABA-A receptor complex / GABA-A receptor activity / innervation ...benzodiazepine receptor activity / GABA receptor complex / cellular response to histamine / GABA receptor activation / inner ear receptor cell development / inhibitory synapse assembly / GABA-gated chloride ion channel activity / GABA-A receptor complex / GABA-A receptor activity / innervation / postsynaptic specialization membrane / gamma-aminobutyric acid signaling pathway / synaptic transmission, GABAergic / adult behavior / chloride channel activity / Signaling by ERBB4 / cochlea development / chloride channel complex / dendrite membrane / chloride transmembrane transport / cytoplasmic vesicle membrane / post-embryonic development / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / GABA-ergic synapse / dendritic spine / chemical synaptic transmission / postsynaptic membrane / postsynapse / axon / extracellular exosome / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
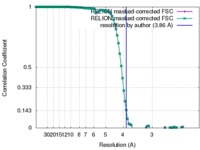
| Method | single particle reconstruction / cryo EM / Resolution: 3.86 Å | |||||||||
 Authors Authors | Zhu S / Noviello CM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2018 Journal: Nature / Year: 2018Title: Structure of a human synaptic GABA receptor. Authors: Shaotong Zhu / Colleen M Noviello / Jinfeng Teng / Richard M Walsh / Jeong Joo Kim / Ryan E Hibbs /  Abstract: Fast inhibitory neurotransmission in the brain is principally mediated by the neurotransmitter GABA (γ-aminobutyric acid) and its synaptic target, the type A GABA receptor (GABA receptor). ...Fast inhibitory neurotransmission in the brain is principally mediated by the neurotransmitter GABA (γ-aminobutyric acid) and its synaptic target, the type A GABA receptor (GABA receptor). Dysfunction of this receptor results in neurological disorders and mental illnesses including epilepsy, anxiety and insomnia. The GABA receptor is also a prolific target for therapeutic, illicit and recreational drugs, including benzodiazepines, barbiturates, anaesthetics and ethanol. Here we present high-resolution cryo-electron microscopy structures of the human α1β2γ2 GABA receptor, the predominant isoform in the adult brain, in complex with GABA and the benzodiazepine site antagonist flumazenil, the first-line clinical treatment for benzodiazepine overdose. The receptor architecture reveals unique heteromeric interactions for this important class of inhibitory neurotransmitter receptor. This work provides a template for understanding receptor modulation by GABA and benzodiazepines, and will assist rational approaches to therapeutic targeting of this receptor for neurological disorders and mental illness. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7816.map.gz emd_7816.map.gz | 5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7816-v30.xml emd-7816-v30.xml emd-7816.xml emd-7816.xml | 28.5 KB 28.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_7816_fsc_1.xml emd_7816_fsc_1.xml emd_7816_fsc_2.xml emd_7816_fsc_2.xml | 9.3 KB 9.3 KB | Display Display |  FSC data file FSC data file |
| Images |  emd_7816_1.png emd_7816_1.png emd_7816_2.png emd_7816_2.png emd_7816_3.png emd_7816_3.png emd_7816_4.png emd_7816_4.png | 183.3 KB 186.2 KB 205.5 KB 198.8 KB | ||
| Filedesc metadata |  emd-7816.cif.gz emd-7816.cif.gz | 8 KB | ||
| Others |  emd_7816_additional.map.gz emd_7816_additional.map.gz emd_7816_half_map_1.map.gz emd_7816_half_map_1.map.gz emd_7816_half_map_2.map.gz emd_7816_half_map_2.map.gz | 4.5 MB 51.9 MB 51.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7816 http://ftp.pdbj.org/pub/emdb/structures/EMD-7816 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7816 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7816 | HTTPS FTP |
-Related structure data
| Related structure data |  6d6tMC  7817C  6d6uC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7816.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7816.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map sharpened to B factor -153 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
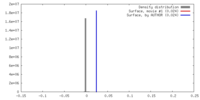
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Main map sharpened to B factor -100
| File | emd_7816_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map sharpened to B factor -100 | ||||||||||||
| Projections & Slices |
| ||||||||||||
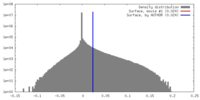
| Density Histograms |
-Half map: half map 1
| File | emd_7816_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
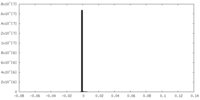
| Density Histograms |
-Half map: half map 2
| File | emd_7816_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
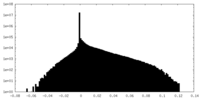
| Density Histograms |
- Sample components
Sample components
+Entire : Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with...
+Supramolecule #1: Human GABA-A receptor alpha1-beta2-gamma2 subtype in complex with...
+Macromolecule #1: Gamma-aminobutyric acid receptor subunit beta-2,Gamma-aminobutyri...
+Macromolecule #2: Gamma-aminobutyric acid receptor subunit alpha-1,Gamma-aminobutyr...
+Macromolecule #3: Human GABA-A receptor subunit gamma-2
+Macromolecule #4: Kappa Fab Light Chain
+Macromolecule #5: IgG2b Fab Heavy Chain
+Macromolecule #9: 2-acetamido-2-deoxy-beta-D-glucopyranose
+Macromolecule #10: GAMMA-AMINO-BUTANOIC ACID
+Macromolecule #11: CHOLESTEROL HEMISUCCINATE
+Macromolecule #12: ethyl 8-fluoro-5-methyl-6-oxo-5,6-dihydro-4H-imidazo[1,5-a][1,4]b...
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 6 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| |||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 80 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 39.0 kPa Details: Sample was glow discharged at 30 mA for 80 seconds using a PELCO easiGLow | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 3 seconds blot time. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 80.0 K / Max: 90.0 K |
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Lower energy threshold: -10 eV / Energy filter - Upper energy threshold: 10 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number grids imaged: 2 / Number real images: 5594 / Average exposure time: 10.0 sec. / Average electron dose: 47.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 46730 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-6d6t: |
 Movie
Movie Controller
Controller


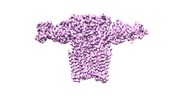
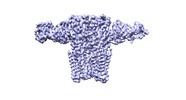










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)