[English] 日本語
 Yorodumi
Yorodumi- EMDB-7621: Cryo-EM structure at 4.0 A resolution of vaccine-elicited antibod... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7621 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure at 4.0 A resolution of vaccine-elicited antibody vFP7.04 in complex with HIV-1 Env BG505 DS-SOSIP, and antibodies VRC03 and PGT122 | |||||||||
 Map data Map data | Map from RELION postprocess using tight mask | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HIV-1 Env / BG505 SOSIP / fusion peptide / VRC03 / PGT122 / vFP7.04 / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell ...symbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.0 Å | |||||||||
 Authors Authors | Acharya P / Carragher B / Potter CS / Kwong PD | |||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2018 Journal: PLoS Pathog / Year: 2018Title: Complete functional mapping of infection- and vaccine-elicited antibodies against the fusion peptide of HIV. Authors: Adam S Dingens / Priyamvada Acharya / Hugh K Haddox / Reda Rawi / Kai Xu / Gwo-Yu Chuang / Hui Wei / Baoshan Zhang / John R Mascola / Bridget Carragher / Clinton S Potter / Julie Overbaugh / ...Authors: Adam S Dingens / Priyamvada Acharya / Hugh K Haddox / Reda Rawi / Kai Xu / Gwo-Yu Chuang / Hui Wei / Baoshan Zhang / John R Mascola / Bridget Carragher / Clinton S Potter / Julie Overbaugh / Peter D Kwong / Jesse D Bloom /  Abstract: Eliciting broadly neutralizing antibodies (bnAbs) targeting envelope (Env) is a major goal of HIV vaccine development, but cross-clade breadth from immunization has only sporadically been observed. ...Eliciting broadly neutralizing antibodies (bnAbs) targeting envelope (Env) is a major goal of HIV vaccine development, but cross-clade breadth from immunization has only sporadically been observed. Recently, Xu et al (2018) elicited cross-reactive neutralizing antibody responses in a variety of animal models using immunogens based on the epitope of bnAb VRC34.01. The VRC34.01 antibody, which was elicited by natural human infection, targets the N terminus of the Env fusion peptide, a critical component of the virus entry machinery. Here we precisely characterize the functional epitopes of VRC34.01 and two vaccine-elicited murine antibodies by mapping all single amino-acid mutations to the BG505 Env that affect viral neutralization. While escape from VRC34.01 occurred via mutations in both fusion peptide and distal interacting sites of the Env trimer, escape from the vaccine-elicited antibodies was mediated predominantly by mutations in the fusion peptide. Cryo-electron microscopy of four vaccine-elicited antibodies in complex with Env trimer revealed focused recognition of the fusion peptide and provided a structural basis for development of neutralization breadth. Together, these functional and structural data suggest that the breadth of vaccine-elicited antibodies targeting the fusion peptide can be enhanced by specific interactions with additional portions of Env. Thus, our complete maps of viral escape both delineate pathways of resistance to these fusion peptide-directed antibodies and provide a strategy to improve the breadth or potency of future vaccine-induced antibodies against Env's fusion peptide. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7621.map.gz emd_7621.map.gz | 14.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7621-v30.xml emd-7621-v30.xml emd-7621.xml emd-7621.xml | 23.6 KB 23.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7621.png emd_7621.png | 129.9 KB | ||
| Filedesc metadata |  emd-7621.cif.gz emd-7621.cif.gz | 6.9 KB | ||
| Others |  emd_7621_additional.map.gz emd_7621_additional.map.gz | 18.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7621 http://ftp.pdbj.org/pub/emdb/structures/EMD-7621 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7621 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7621 | HTTPS FTP |
-Related structure data
| Related structure data |  6cueMC  7622C  6cufC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7621.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7621.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map from RELION postprocess using tight mask | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Map from RELION postprocess using loose mask
| File | emd_7621_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map from RELION postprocess using loose mask | ||||||||||||
| Projections & Slices |
| ||||||||||||
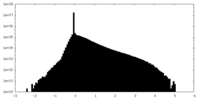
| Density Histograms |
- Sample components
Sample components
+Entire : vFP7.04-BG505 DS-SOSIP-VRC03-PGT122
+Supramolecule #1: vFP7.04-BG505 DS-SOSIP-VRC03-PGT122
+Supramolecule #2: PGT122
+Supramolecule #3: VRC03
+Supramolecule #4: Glycoprotein
+Supramolecule #5: vFP7.04
+Macromolecule #1: Envelope glycoprotein gp120
+Macromolecule #2: Envelope glycoprotein gp41
+Macromolecule #3: vFP7.04 Heavy chain
+Macromolecule #4: vFP7.04 light chain
+Macromolecule #5: PGT122 heavy Chain
+Macromolecule #6: PGT122 Light chain
+Macromolecule #7: VRC03 Heavy chain
+Macromolecule #8: VRC03 light chain
+Macromolecule #14: 2-acetamido-2-deoxy-beta-D-glucopyranose
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7 |
| Vitrification | Cryogen name: ETHANE / Instrument: SPOTITON |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-40 / Number grids imaged: 1 / Number real images: 978 / Average electron dose: 53.11 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 3.0 µm / Calibrated defocus min: 1.5 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 142 / Target criteria: Correlation Coefficient |
|---|---|
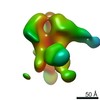
| Output model |  PDB-6cue: |
 Movie
Movie Controller
Controller


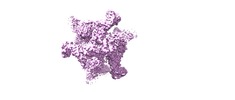









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)