[English] 日本語
 Yorodumi
Yorodumi- EMDB-7603: SARS spike glycoprotein, stabilized variant, S1 CTD configuration... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7603 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SARS spike glycoprotein, stabilized variant, S1 CTD configurations: ACE2-bound, upwards, upwards | |||||||||
 Map data Map data | SARS Spike Glycoprotein, Stabilized variant, single upwards S1 CTD conformation | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationMaturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / positive regulation of amino acid transport / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / Hydrolases; Acting on peptide bonds (peptidases); Metallocarboxypeptidases / angiotensin-mediated drinking behavior / viral translation / regulation of systemic arterial blood pressure by renin-angiotensin ...Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / positive regulation of amino acid transport / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / Hydrolases; Acting on peptide bonds (peptidases); Metallocarboxypeptidases / angiotensin-mediated drinking behavior / viral translation / regulation of systemic arterial blood pressure by renin-angiotensin / positive regulation of gap junction assembly / tryptophan transport / regulation of cardiac conduction / maternal process involved in female pregnancy / peptidyl-dipeptidase activity / regulation of vasoconstriction / transporter activator activity / Metabolism of Angiotensinogen to Angiotensins / carboxypeptidase activity / angiotensin maturation / viral life cycle / Attachment and Entry / receptor-mediated endocytosis of virus by host cell / metallocarboxypeptidase activity / positive regulation of cardiac muscle contraction / regulation of cytokine production / blood vessel diameter maintenance / negative regulation of smooth muscle cell proliferation / brush border membrane / negative regulation of ERK1 and ERK2 cascade / positive regulation of reactive oxygen species metabolic process / metallopeptidase activity / SARS-CoV-1 activates/modulates innate immune responses / endocytic vesicle membrane / regulation of cell population proliferation / virus receptor activity / regulation of inflammatory response / endopeptidase activity / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / Potential therapeutics for SARS / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / apical plasma membrane / host cell surface receptor binding / cilium / symbiont-mediated suppression of host innate immune response / membrane raft / endocytosis involved in viral entry into host cell / endoplasmic reticulum lumen / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / host cell plasma membrane / virion membrane / cell surface / negative regulation of transcription by RNA polymerase II / extracellular space / extracellular exosome / extracellular region / zinc ion binding / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  SARS coronavirus / SARS coronavirus /  Homo sapiens (human) Homo sapiens (human) | |||||||||
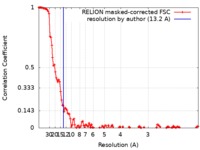
| Method | single particle reconstruction / cryo EM / Resolution: 13.2 Å | |||||||||
 Authors Authors | Kirchdoerfer RN / Wang N / Pallesen J / Turner HL / Cottrell CA / McLellan JS / Ward AB | |||||||||
 Citation Citation |  Journal: Sci Rep / Year: 2018 Journal: Sci Rep / Year: 2018Title: Stabilized coronavirus spikes are resistant to conformational changes induced by receptor recognition or proteolysis. Authors: Robert N Kirchdoerfer / Nianshuang Wang / Jesper Pallesen / Daniel Wrapp / Hannah L Turner / Christopher A Cottrell / Kizzmekia S Corbett / Barney S Graham / Jason S McLellan / Andrew B Ward /  Abstract: Severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in 2002 as a highly transmissible pathogenic human betacoronavirus. The viral spike glycoprotein (S) utilizes angiotensin-converting ...Severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in 2002 as a highly transmissible pathogenic human betacoronavirus. The viral spike glycoprotein (S) utilizes angiotensin-converting enzyme 2 (ACE2) as a host protein receptor and mediates fusion of the viral and host membranes, making S essential to viral entry into host cells and host species tropism. As SARS-CoV enters host cells, the viral S is believed to undergo a number of conformational transitions as it is cleaved by host proteases and binds to host receptors. We recently developed stabilizing mutations for coronavirus spikes that prevent the transition from the pre-fusion to post-fusion states. Here, we present cryo-EM analyses of a stabilized trimeric SARS-CoV S, as well as the trypsin-cleaved, stabilized S, and its interactions with ACE2. Neither binding to ACE2 nor cleavage by trypsin at the S1/S2 cleavage site impart large conformational changes within stabilized SARS-CoV S or expose the secondary cleavage site, S2'. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7603.map.gz emd_7603.map.gz | 163 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7603-v30.xml emd-7603-v30.xml emd-7603.xml emd-7603.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_7603_fsc.xml emd_7603_fsc.xml | 13 KB | Display |  FSC data file FSC data file |
| Images |  emd_7603.png emd_7603.png | 75.3 KB | ||
| Masks |  emd_7603_msk_1.map emd_7603_msk_1.map | 178 MB |  Mask map Mask map | |
| Others |  emd_7603_half_map_1.map.gz emd_7603_half_map_1.map.gz emd_7603_half_map_2.map.gz emd_7603_half_map_2.map.gz | 140.3 MB 140.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7603 http://ftp.pdbj.org/pub/emdb/structures/EMD-7603 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7603 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7603 | HTTPS FTP |
-Related structure data
| Related structure data |  7573C  7574C  7575C  7576C  7577C  7578C  7579C  7580C  7581C  7582C  7584C  7585C  7586C  7601C  7602C  7604C  7605C  7606C  7607C  7608C  6crvC  6crwC  6crxC  6crzC  6cs0C  6cs1C  6cs2C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7603.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7603.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | SARS Spike Glycoprotein, Stabilized variant, single upwards S1 CTD conformation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
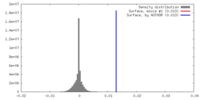
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_7603_msk_1.map emd_7603_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: SARS Spike Glycoprotein, Stabilized variant, single upwards S1...
| File | emd_7603_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | SARS Spike Glycoprotein, Stabilized variant, single upwards S1 CTD conformation | ||||||||||||
| Projections & Slices |
| ||||||||||||
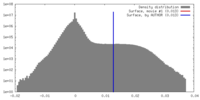
| Density Histograms |
-Half map: SARS Spike Glycoprotein, Stabilized variant, single upwards S1...
| File | emd_7603_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | SARS Spike Glycoprotein, Stabilized variant, single upwards S1 CTD conformation | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : SARS Spike Glycoprotein complexed to ACE2 receptor
| Entire | Name: SARS Spike Glycoprotein complexed to ACE2 receptor |
|---|---|
| Components |
|
-Supramolecule #1: SARS Spike Glycoprotein complexed to ACE2 receptor
| Supramolecule | Name: SARS Spike Glycoprotein complexed to ACE2 receptor / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  SARS coronavirus / Strain: Tor2 SARS coronavirus / Strain: Tor2 |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK293F Homo sapiens (human) / Recombinant cell: HEK293F |
| Molecular weight | Theoretical: 620 KDa |
-Macromolecule #1: SARS spike glycoprotein
| Macromolecule | Name: SARS spike glycoprotein / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  SARS coronavirus / Strain: Tor2 SARS coronavirus / Strain: Tor2 |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: SDLDRCTTFD DVQAPNYTQH TSSMRGVYYP DEIFRSDTLY LTQDLFLPFY SNVTGFHTIN HTFGNPVIPF KDGIYFAATE KSNVVRGWVF GSTMNNKSQS VIIINNSTNV VIRACNFELC DNPFFAVSKP MGTQTHTMIF DNAFNCTFEY ISDAFSLDVS EKSGNFKHLR ...String: SDLDRCTTFD DVQAPNYTQH TSSMRGVYYP DEIFRSDTLY LTQDLFLPFY SNVTGFHTIN HTFGNPVIPF KDGIYFAATE KSNVVRGWVF GSTMNNKSQS VIIINNSTNV VIRACNFELC DNPFFAVSKP MGTQTHTMIF DNAFNCTFEY ISDAFSLDVS EKSGNFKHLR EFVFKNKDGF LYVYKGYQPI DVVRDLPSGF NTLKPIFKLP LGINITNFRA ILTAFSPAQD IWGTSAAAYF VGYLKPTTFM LKYDENGTIT DAVDCSQNPL AELKCSVKSF EIDKGIYQTS NFRVVPSGDV VRFPNITNLC PFGEVFNATK FPSVYAWERK KISNCVADYS VLYNSTFFST FKCYGVSATK LNDLCFSNVY ADSFVVKGDD VRQIAPGQTG VIADYNYKLP DDFMGCVLAW NTRNIDATST GNYNYKYRYL RHGKLRPFER DISNVPFSPD GKPCTPPALN CYWPLNDYGF YTTTGIGYQP YRVVVLSFEL LNAPATVCGP KLSTDLIKNQ CVNFNFNGLT GTGVLTPSSK RFQPFQQFGR DVSDFTDSVR DPKTSEILDI SPCAFGGVSV ITPGTNASSE VAVLYQDVNC TDVSTAIHAD QLTPAWRIYS TGNNVFQTQA GCLIGAEHVD TSYECDIPIG AGICASYHTV SLLRSTSQKS IVAYTMSLGA DSSIAYSNNT IAIPTNFSIS ITTEVMPVSM AKTSVDCNMY ICGDSTECAN LLLQYGSFCT QLNRALSGIA AEQDRNTREV FAQVKQMYKT PTLKYFGGFN FSQILPDPLK PTKRSFIEDL LFNKVTLADA GFMKQYGECL GDINARDLIC AQKFNGLTVL PPLLTDDMIA AYTAALVSGT ATAGWTFGAG AALQIPFAMQ MAYRFNGIGV TQNVLYENQK QIANQFNKAI SQIQESLTTT STALGKLQDV VNQNAQALNT LVKQLSSNFG AISSVLNDIL SRLDPPEAEV QIDRLITGRL QSLQTYVTQQ LIRAAEIRAS ANLAATKMSE CVLGQSKRVD FCGKGYHLMS FPQAAPHGVV FLHVTYVPSQ ERNFTTAPAI CHEGKAYFPR EGVFVFNGTS WFITQRNFFS PQIITTDNTF VSGNCDVVIG IINNTVYDPL QPELDSFKEE LDKYFKNHTS PDVDLGDISG INASVVNIQK EIDRLNEVAK NLNESLIDLQ ELGKYEQGSG YIPEAPRDGQ AYVRKDGEWV LLSTFLGRSL EVLFQ |
-Macromolecule #2: Angiotensin converting enzyme 2
| Macromolecule | Name: Angiotensin converting enzyme 2 / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: STIEEQAKTF LDKFNHEAED LFYQSSLASW NYNTNITEEN VQNMNNAGDK WSAFLKEQST LAQMYPLQEI QNLTVKLQLQ ALQQNGSSVL SEDKSKRLNT ILNTMSTIYS TGKVCNPDNP QECLLLEPGL NEIMANSLDY NERLWAWESW RSEVGKQLRP LYEEYVVLKN ...String: STIEEQAKTF LDKFNHEAED LFYQSSLASW NYNTNITEEN VQNMNNAGDK WSAFLKEQST LAQMYPLQEI QNLTVKLQLQ ALQQNGSSVL SEDKSKRLNT ILNTMSTIYS TGKVCNPDNP QECLLLEPGL NEIMANSLDY NERLWAWESW RSEVGKQLRP LYEEYVVLKN EMARANHYED YGDYWRGDYE VNGVDGYDYS RGQLIEDVEH TFEEIKPLYE HLHAYVRAKL MNAYPSYISP IGCLPAHLLG DMWGRFWTNL YSLTVPFGQK PNIDVTDAMV DQAWDAQRIF KEAEKFFVSV GLPNMTQGFW ENSMLTDPGN VQKAVCHPTA WDLGKGDFRI LMCTKVTMDD FLTAHHEMGH IQYDMAYAAQ PFLLRNGANE GFHEAVGEIM SLSAATPKHL KSIGLLSPDF QEDNETEINF LLKQALTIVG TLPFTYMLEK WRWMVFKGEI PKDQWMKKWW EMKREIVGVV EPVPHDETYC DPASLFHVSN DYSFIRYYTR TLYQFQFQEA LCQAAKHEGP LHKCDISNST EAGQKLFNML RLGKSEPWTL ALENVVGAKN MNVRPLLNYF EPLFTWLKDQ NKNSFVGWST DWSPYADGSL EVLFQ |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.35 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: C-flat-2/2 4C / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Atmosphere: OTHER | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Average exposure time: 0.25 sec. / Average electron dose: 65.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)