[English] 日本語
 Yorodumi
Yorodumi- EMDB-71924: Cryo-EM Structure of Pig Ryanodine Receptor 1 R615C Mutant: Atorv... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of Pig Ryanodine Receptor 1 R615C Mutant: Atorvastatin Bound Open Conformation Composite Map | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ion Channel / Calcium Channel / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of sequestering of calcium ion / negative regulation of calcium-mediated signaling / negative regulation of insulin secretion involved in cellular response to glucose stimulus / neuronal action potential propagation / negative regulation of release of sequestered calcium ion into cytosol / insulin secretion involved in cellular response to glucose stimulus / terminal cisterna / ryanodine-sensitive calcium-release channel activity / ryanodine receptor complex / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum ...positive regulation of sequestering of calcium ion / negative regulation of calcium-mediated signaling / negative regulation of insulin secretion involved in cellular response to glucose stimulus / neuronal action potential propagation / negative regulation of release of sequestered calcium ion into cytosol / insulin secretion involved in cellular response to glucose stimulus / terminal cisterna / ryanodine-sensitive calcium-release channel activity / ryanodine receptor complex / release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / response to redox state / ossification involved in bone maturation / negative regulation of heart rate / cellular response to caffeine / 'de novo' protein folding / skin development / FK506 binding / organelle membrane / smooth endoplasmic reticulum / outflow tract morphogenesis / smooth muscle contraction / T cell proliferation / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / voltage-gated calcium channel activity / calcium channel inhibitor activity / skeletal muscle fiber development / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / Ion homeostasis / release of sequestered calcium ion into cytosol / calcium channel complex / sarcoplasmic reticulum membrane / cellular response to calcium ion / muscle contraction / sarcoplasmic reticulum / protein maturation / calcium channel regulator activity / peptidylprolyl isomerase / peptidyl-prolyl cis-trans isomerase activity / calcium-mediated signaling / sarcolemma / calcium ion transmembrane transport / Stimuli-sensing channels / calcium channel activity / Z disc / intracellular calcium ion homeostasis / positive regulation of cytosolic calcium ion concentration / protein refolding / protein homotetramerization / transmembrane transporter binding / calmodulin binding / signaling receptor binding / calcium ion binding / ATP binding / membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.36 Å | |||||||||
 Authors Authors | Molinarolo SM / Van Petegem F | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Cryo-electron microscopy reveals sequential binding and activation of Ryanodine Receptors by statin triplets. Authors: Steven Molinarolo / Carmen R Valdivia / Héctor H Valdivia / Filip Van Petegem /   Abstract: Statins are the most prescribed class of drugs and inhibit a key enzyme in the cholesterol biosynthesis pathway. Many patients have reported mild to severe muscle related symptoms and a subset are at ...Statins are the most prescribed class of drugs and inhibit a key enzyme in the cholesterol biosynthesis pathway. Many patients have reported mild to severe muscle related symptoms and a subset are at risk for rhabdomyolysis. Sequence variants in RyR1, the skeletal muscle Ryanodine Receptor, correlate with intolerance to statins, but whether RyR1 can bind statins directly has remained unclear. Here we report cryo-EM structures of RyR1 in the absence and presence of atorvastatin, firmly establishing RyR1 as an unintended off-target. Our results show an unusual binding mode whereby three atorvastatin molecules bind together in a cleft formed by the pseudo-voltage sensing domain, making extensive interactions with each other and with RyR1. Atorvastatin activates RyR1 in a sequential way, whereby one statin per subunit can bind to the transmembrane region of a closed RyR1, with small structural perturbations that prime the channel for opening. Binding of two additional statins per subunit is associated with a widening of the pseudo-voltage sensing domain that triggers opening of the pore. Comparison with atorvastatin binding to HMG-CoA reductase, its intended target, offers clues on how to modify the statin to reduce RyR1 binding, while leaving binding to HMG-CoA reductase unperturbed. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_71924.map.gz emd_71924.map.gz | 48 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-71924-v30.xml emd-71924-v30.xml emd-71924.xml emd-71924.xml | 26.6 KB 26.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_71924.png emd_71924.png | 145.1 KB | ||
| Filedesc metadata |  emd-71924.cif.gz emd-71924.cif.gz | 10.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-71924 http://ftp.pdbj.org/pub/emdb/structures/EMD-71924 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-71924 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-71924 | HTTPS FTP |
-Validation report
| Summary document |  emd_71924_validation.pdf.gz emd_71924_validation.pdf.gz | 447.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_71924_full_validation.pdf.gz emd_71924_full_validation.pdf.gz | 447.4 KB | Display | |
| Data in XML |  emd_71924_validation.xml.gz emd_71924_validation.xml.gz | 7.9 KB | Display | |
| Data in CIF |  emd_71924_validation.cif.gz emd_71924_validation.cif.gz | 9.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-71924 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-71924 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-71924 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-71924 | HTTPS FTP |
-Related structure data
| Related structure data |  9pwoMC  9ol6C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_71924.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_71924.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.96 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Ryanodine Receptor 1 and FKBP12.6 complex
| Entire | Name: Ryanodine Receptor 1 and FKBP12.6 complex |
|---|---|
| Components |
|
-Supramolecule #1: Ryanodine Receptor 1 and FKBP12.6 complex
| Supramolecule | Name: Ryanodine Receptor 1 and FKBP12.6 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 2.39 MDa |
-Macromolecule #1: Ryanodine receptor 1
| Macromolecule | Name: Ryanodine receptor 1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 565.935062 KDa |
| Sequence | String: MGDGGEGEDE VQFLRTDDEV VLQCNATVLK EQLKLCLAAE GFGNRLCFLE PTSNAQNVPP DLAICCFVLE QSLSVRALQE MLANTVEAG VESSQGGGHR TLLYGHAILL RHAHSGMYLS CLTTSRSMTD KLAFDVGLQE DATGEACWWT THPASKQRSE G EKVRVGDD ...String: MGDGGEGEDE VQFLRTDDEV VLQCNATVLK EQLKLCLAAE GFGNRLCFLE PTSNAQNVPP DLAICCFVLE QSLSVRALQE MLANTVEAG VESSQGGGHR TLLYGHAILL RHAHSGMYLS CLTTSRSMTD KLAFDVGLQE DATGEACWWT THPASKQRSE G EKVRVGDD LILVSVSSER YLHLSTASGE LQVDASFMQT LWNMNPICSG CEEGYVTGGH VLRLFHGHMD ECLTISPADS DD QRRLVYY EGGSVCTHAR SLWRLEPLRI SWSGSHLRWG QPLRIRHVTT GRYLALIEDQ GLVVVDASKA HTKATSFCFR ISK EKLDTA PKRDVEGMGP PEIKYGESLC FVQHVASGLW LTYAAPDPKA LRLGVLKKKA ILHQEGHMDD ALSLTRCQQE ESQA ARMIY STAGLYNHFI KGLDSFSGKP RGSGAPAGTA LPLEGVILSL QDLIGYFEPP SEELQHEEKQ SKLRSLRNRQ SLFQE EGML SLVLNCIDRL NVYTTAAHFA EFAGEEAAES WKEIVNLLYE ILASLIRGNR ANCALFSNNL DWLVSKLDRL EASSGI LEV LYCVLIESPE VLNIIQENHI KSIISLLDKH GRNHKVLDVL CSLCVCNGVA VCSNQDLITE NLLPGRELLL QTNLINY VT SIRPNIFVGR AEGTTQYSKW YFEVMVDEVV PFLTAQATHL RVGWALTEGY SPYPGGGEGW GGNGVGDDLY SYGFDGLH L WTGHVPRLVT SPGQHLLAPE DVVSCCLDLS VPSISFRING CPVQGVFEAF NLNGLFFPVV SFSAGVKVRF LLGGRHGEF KFLPPPGYAP CHEAVLPRER LRLEPIKEYR REGPRGPHLV GPSRCLSHTD FVPCPVDTVQ IVLPPHLERI REKLAENIHE LWALTRIEQ GWTYGPVRDD NKRLHPCLVD FHSLPEPERN YNLQMSGETL KTLLALGCHV GMADEKAEDN LRKTKLPKTY M MSNGYKPA PLDLSHVRLT PAQTTLVDRL AENGHNVWAR DRVAQGWSYS AVQDIPARRN PRLVPYRLLD EATKRSNRDS LC QAVRTLL GYGYNIEPPD QEPSQVESQS RWDRVRIFRA EKSYAVQSGR WYFEFEAVTT GEMRVGWARP ELRPDVELGA DEL AYVFNG HRGQRWHLGS ELFGRPWQSG DVVGCMIDLT ENTIIFTLNG EVLMSDSGSE TAFRDIEVGD GFLPVCSLGP GQVG HLNLG QDVSSLRFFA ICGLQEGFEP FAINMQRPVT TWFSKSLPQF EAVPLEHPHY EVSRVDGTVD TPPCLRLTHR TWGSQ NSLV EMLFLRLSLP VQFHQHFRCT AGATPLAPPG LQPPAEDEAR AAEPDPDYEN LRRSAGRWGE AEGGKEGTAK EGAPGG TAQ AGVEAQPPRA ENEKDATTEK NKKRGFLFKA KKAAMMTQPP ATPTLPRLPH EVVPADDRDD PDIILNTTTY YYSVRVF AG QEPSCVWVGW VTPDYHQHDM NFDLTKVRAV TVTMGDEQGN IHSSLKCSNC YMVWGGDFVS PGQQGRISHT DLVIGCLV D LATGLMTFTA NGKESNTFFQ VEPNTKLFPA VFVLPTHQNV IQFELGKQKN IMPLSAAMFL SERKNPAPQC PPRLEMQML MPVSWSRMPN HFLRVETRRA GERLGWAVQC QEPLTMMALH IPEENRCMDI LELSERLDLQ QFHSHTLRLY RAVCALGNNR VAHALCSHV DQAQLLHALE DAHLPGPLRA GYYDLLISIH LESACRSRRS MLSEYIVPLT PETRAITLFP PGKRTENGPR R HGLPGVGV TTSLRPPHHF SAPCFVAALP AVGAAEAPAR LSPSIPLEAL RDKALRMLGE AVRDGGQHAR DPVGGSVEFQ FV PVLKLVS TLLVMGIFGD EDVKQILKMI EPEVFTEEEE EEEEEEEEEE EDEEEKEEDE EEEAREKEDE EKEEEETAEG EKE EYLEEG LLQMKLPESV KLQMCNLLEY FCDQELQHRV ESLAAFAERY VDKLQANQRD RYGILMKAFT MTAAETARRT REFR SPPQE QINMLLHFKD GEDEEDCPLP DEIRQDLLEF HQDLLTHCGI QLEGEEEEPE EEATLGSRLM SLLEKVRLVK KKEEK SEEE PPAEESKAQS LQELVSHTVV RWAQEDFVQS PELVRAMFSL LHRQYDGLGE LLRALPRAYT ISPSSVEDTM SLLECL GQI RSLLIVQMGP QEENLMIQSI GNIMNNKVFY QHPNLMRALG MHETVMEVMV NVLGGGESKE IRFPKMVTSC CRFLCYF CR ISRQNQRSMF DHLSYLLENS GIGLGMQGST PLDVAAASVI DNNELALALQ EQDLEKVVSY LAGCGLQSCP MLLAKGYP D IGWNPCGGER YLDFLRFAVF VNGESVEENA NVVVRLLIRK PECFGPALRG EGGSGLLATI EEAIRISEDP ARDGPGVRR DRRREHFGEE PPEENRVHLG HAIMSFYAAL IDLLGRCAPE MHLIQAGKGE ALRIRAILRS LVPLDDLVGI ISLPLQIPTL GKDGALVQP KMSASFVPDH KASMVLFLDR VYGIENQDFL LHVLDVGFLP DMRAAASLDT ATFSTTEMAL ALNRYLCLAV L PLITKCAP LFAGTEHRAI MVDSMLHTVY RLSRGRSLTK AQRDVIEECL MALCRYIRPS MLQHLLRRLV FDVPILNEFA KM PLKLLTN HYERCWKYYC LPTGWANFGV TSEEELHLTR KLFWGIFDSL AHKKYDPELY RMAMPCLCAI AGALPPDYVD ASY SSKAEK KATVDAEGNF DPRPVETLNV IIPEKLDSFI NKFAEYTHEK WAFDKIQNNW SYGENIDEEL KTHPMLRPYK TFSE KDKEI YRWPIKESLK AMIAWEWTIE KAREGEEEKT EKKKTRKISQ SAQTYDAREG YNPQPPDLSG VTLSRELQAM AEQLA ENYH NTWGRKKKQE LEAKGGGTHP LLVPYDTLTA KEKARDREKA QELLKFLQMN GYAVTRGLKD MELDTSSIEK RFAFGF LQQ LLRWMDISQE FIAHLEAVVS SGRVEKSPHE QEIKFFAKIL LPLINQYFTN HCLYFLSTPA KVLGSGGHAS NKEKEMI TS LFCKLAALVR HRVSLFGTDA PAVVNCLHIL ARSLDARTVM KSGPEIVKAG LRSFFESASE DIEKMVENLR LGKVSQAR T QVKGVGQNLT YTTVALLPVL TTLFQHIAQH QFGDDVILDD VQVSCYRTLC SIYSLGTTRN PYVEKLRPAL GECLARLAA AMPVAFLEPQ LNEYNACSVY TTKSPRERAI LGLPNSVEEM CPDIPVLERL MADIGGLAES GARYTEMPHV IEITLPMLCS YLPRWWERG PEAPPPALPA GAPPPCTAVT SDHLNSLLGN ILRIIVNNLG IDEASWMKRL AVFAQPIVSR ARPELLHSHF I PTIGRLRK RAGKVVAEEE QLRLEAKAEA EEGELLVRDE FSVLCRDLYA LYPLLIRYVD NNRAHWLTEP NPSAEELFRM VG EIFIYWS KSHNFKREEQ NFVVQNEINN MSFLTADNKS KMAKSGGSDQ ERTKKKRLGD RYSVQTSLIV ATLKKMLPIG LNM CAPTDQ ELITLAKTRY ALKDTDEEVR EFLQNNLHLQ GKVEGSPSLR WQMALYRGLP GREEDADDPE KIVRRVQEVS AVLY HLEQM EHPYKSKKAV WHKLLSKQRR RAVVACFRMT PLYNLPTHRA CNMFLESYKA AWILTEDHSF EDRMIDDLSK AGEQE EEEE EVEEKKPDPL HQLVLHFSRT ALTEKSKLDE DYLYMAYADI MAKSCHLEEG GENGEAQEEV EVSFEEKEME KQRLLY QQA RLHNRGAAEM VLQMISACKG ETGAMVSSTL KLGISILNGG NADVQQKMLD YLKDKKEVGF FQSIQALMQT CSVLDLN AF ERQNKAEGLG MVNEDGTVIN RQNGEKVMAD DEFTQDLFRF LQLLCEGHNN DFQNYLRTQT GNTTTINIII CTVDYLLR L QESISDFYWY YSGKDVIEEQ GKRNFSKAMS VAKQVFNSLT EYIQGPCTGN QQSLAHSRLW DAVVGFLHVF AHMMMKLAQ DSSQIELLKE LLDLQKDMVV MLLSLLEGNV VNGMIARQMV DMLVESSSNV EMILKFFDMF LKLKDIVGSE AFQDYVTDPR GLISKKDFQ KAMDSQKQFT GPEIQFLLSC SEADENEMID CEEFANRFQE PARDIGFNVA VLLTNLSEHV PHDPRLRNFL E LAESILEY FRPYLGRIEI MGASRRIERI YFEISETNRA QWEMPQVKES KRQFIFDVVN EGGESEKMEL FVSFCEDTIF EM QIAAQIS EPEGEPEEDE DEGAGLAEAG AEGAEEGAVG PEGAAGTAAA GLTARLAAAT SRALRGLSYR SLRRRVRRLR RLT AREAAT ALAALLWAAL AHAGAAGAGA AAGALRLLWG SLFGGGLVEG AKKVTVTELL AGMPDPTGDE VHGEQPAGPG GEAD GEGAG EGAGEAWEGA GDEEVAVQEA GPGGADGAVA VAEGGPFRPE GAGGLGDMGD TTPAEPPTPE GSPIIKRKLG VDGEE EELP PEPEPEPEPE PEKADAENGE KEEVPKPPPE PPKKTAPPPP PPKKEEGGSG GLEFWGELEV QRVKFLNYLS RNFYTL RFL ALFLAFAINF ILLFYKVSDS PPGEDDMEGS AAGDLSGAGS GGGSGWGSGA GEEVEGDEDE NMVYYFLEES TGYMEPA LR CLSLLHTLVA FLCIIGYNCL KVPLVIFKRE KELARKLEFD GLYITEQPED DDVKGQWDRL VLNTPSFPSN YWDKFVKR K VLDKHGDIYG RERIAELLGM DLATLEITAH NERKPEPPPG LLTWLMSIDV KYQIWKFGVI FTDNSFLYLG WYMVMSLLG HYNNFFFAAH LLDIAMGVKT LRTILSSVTH NGKQLVMTVG LLAVVVYLYT VVAFNFFRKF YNKSEDEDEP DMKCDDMMTC YLFHMYVGV RAGGGIGDEI EDPAGDEYEL YRVVFDITFF FFVIVILLAI IQGLIIDAFG ELRDQQEQVR EDMETKCFIC G IGSDYFDT TPHRFETHTL EEHNLANYMF FLMYLINKDE TEHTGQESYV WKMYQERCWD FFPAGDCFRK QYEDQLS UniProtKB: Ryanodine receptor 1 |
-Macromolecule #2: Peptidyl-prolyl cis-trans isomerase FKBP1B
| Macromolecule | Name: Peptidyl-prolyl cis-trans isomerase FKBP1B / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO / EC number: peptidylprolyl isomerase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.070759 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SNAMGVEIET ISPGDGRTFP KKGQTCVVHY TGMLQNGKKF DSSRDRNKPF KFRIGKQEVI KGFEEGAAQM SLGQRAKLTC TPDVAYGAT GHPGVIPPNA TLIFDVELLN LE UniProtKB: Peptidyl-prolyl cis-trans isomerase FKBP1B |
-Macromolecule #3: CAFFEINE
| Macromolecule | Name: CAFFEINE / type: ligand / ID: 3 / Number of copies: 4 / Formula: CFF |
|---|---|
| Molecular weight | Theoretical: 194.191 Da |
| Chemical component information |  ChemComp-CFF: |
-Macromolecule #4: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 4 / Number of copies: 4 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #5: 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYR...
| Macromolecule | Name: 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL]- 3,5-DIHYDROXY-HEPTANOIC ACID type: ligand / ID: 5 / Number of copies: 12 / Formula: 117 |
|---|---|
| Molecular weight | Theoretical: 558.64 Da |
| Chemical component information | 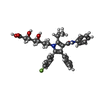 ChemComp-117: |
-Macromolecule #6: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 6 / Number of copies: 4 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 9 mg/mL | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 1 / Number real images: 5007 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















