+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of G6PT1 bound with GlcN6P | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | G6P / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationglucose-6-phosphate transmembrane transporter activity / Glycogen storage disease type Ib (SLC37A4) / glucose 6-phosphate:phosphate antiporter activity / glucose-6-phosphate transport / Gluconeogenesis / phosphate ion transmembrane transport / gluconeogenesis / glucose metabolic process / glucose homeostasis / endoplasmic reticulum membrane ...glucose-6-phosphate transmembrane transporter activity / Glycogen storage disease type Ib (SLC37A4) / glucose 6-phosphate:phosphate antiporter activity / glucose-6-phosphate transport / Gluconeogenesis / phosphate ion transmembrane transport / gluconeogenesis / glucose metabolic process / glucose homeostasis / endoplasmic reticulum membrane / endoplasmic reticulum / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Shuai G / Xia Y / Qian W | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Structures of human glucose-6-phosphate transporter reveal reciprocal antiport mechanism driving glucose-6-phosphate and inorganic phosphate exchange. Authors: Qian Wang / Ningjie Guo / Yunxiang Du / Junwu Liu / Wanting Ai / Fengyi Yang / Yuanzai Zhu / Yuanyuan Zhao / Di Wu / Lei Liu / Xia Yao / Shuai Gao /  Abstract: Glucose-6-phosphate transporter 1 (G6PT1) is essential for systemic glucose homeostasis, and its deficiency causes glycogen storage disease type 1b (GSD1b). G6PT1 functions as a sugar- ...Glucose-6-phosphate transporter 1 (G6PT1) is essential for systemic glucose homeostasis, and its deficiency causes glycogen storage disease type 1b (GSD1b). G6PT1 functions as a sugar-phosphate/inorganic phosphate (Pi) antiporter, orchestrating G6P transport into the endoplasmic reticulum lumen driven by a Pi gradient. Despite its physiological significance, the molecular mechanisms underlying substrate recognition and antiport activity remain poorly characterized. Here, we present cryo-electron microscopy structures of human G6PT1 in apo, Pi-bound, and GlcN6P-bound (a G6P analogue) states, all captured in cytosol-open conformations. Combined with molecular docking and functional assays, these structures elucidate the molecular basis for Pi and G6P recognition in G6PT1. Comparative analysis reveals that Pi binding triggers an interdomain salt bridge formation, resulting in a thicker luminal gate and a more compact central cavity for G6P binding. In addition to the monomer, we identify a dimeric assembly of G6PT1. Mutating key residues at the dimer interface impairs transport activity, suggesting a regulatory role for oligomerization. Our findings thus provide a mechanistic framework for understanding G6PT1 working mechanism and its pathological dysregulation in GSD1b. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_64148.map.gz emd_64148.map.gz | 49.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-64148-v30.xml emd-64148-v30.xml emd-64148.xml emd-64148.xml | 17.7 KB 17.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_64148.png emd_64148.png | 40.4 KB | ||
| Filedesc metadata |  emd-64148.cif.gz emd-64148.cif.gz | 5.9 KB | ||
| Others |  emd_64148_half_map_1.map.gz emd_64148_half_map_1.map.gz emd_64148_half_map_2.map.gz emd_64148_half_map_2.map.gz | 48.9 MB 48.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-64148 http://ftp.pdbj.org/pub/emdb/structures/EMD-64148 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-64148 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-64148 | HTTPS FTP |
-Related structure data
| Related structure data |  9ugyMC  9uguC  9ugxC  9ws8C  9wt2C  9x8xC  9x95C  9x97C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_64148.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_64148.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.824 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_64148_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_64148_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : G6PT1
| Entire | Name: G6PT1 |
|---|---|
| Components |
|
-Supramolecule #1: G6PT1
| Supramolecule | Name: G6PT1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Glucose-6-phosphate exchanger SLC37A4
| Macromolecule | Name: Glucose-6-phosphate exchanger SLC37A4 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 46.391809 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAAQGYGYYR TVIFSAMFGG YSLYYFNRKT FSFVMPSLVE EIPLDKDDLG FITSSQSAAY AISKFVSGVL SDQMSARWLF SSGLLLVGL VNIFFAWSST VPVFAALWFL NGLAQGLGWP PCGKVLRKWF EPSQFGTWWA ILSTSMNLAG GLGPILATIL A QSYSWRST ...String: MAAQGYGYYR TVIFSAMFGG YSLYYFNRKT FSFVMPSLVE EIPLDKDDLG FITSSQSAAY AISKFVSGVL SDQMSARWLF SSGLLLVGL VNIFFAWSST VPVFAALWFL NGLAQGLGWP PCGKVLRKWF EPSQFGTWWA ILSTSMNLAG GLGPILATIL A QSYSWRST LALSGALCVV VSFLCLLLIH NEPADVGLRN LDPMPSEGKK GSLKEESTLQ ELLLSPYLWV LSTGYLVVFG VK TCCTDWG QFFLIQEKGQ SALVGSSYMS ALEVGGLVGS IAAGYLSDRA MAKAGLSNYG NPRHGLLLFM MAGMTVSMYL FRV TVTSDS PKLWILVLGA VFGFSSYGPI ALFGVIANES APPNLCGTSH AIVGLMANVG GFLAGLPFST IAKHYSWSTA FWVA EVICA ASTAAFFLLR NIRTKMGRVS KKAE UniProtKB: Glucose-6-phosphate exchanger SLC37A4 |
-Macromolecule #2: 2-amino-2-deoxy-6-O-phosphono-alpha-D-glucopyranose
| Macromolecule | Name: 2-amino-2-deoxy-6-O-phosphono-alpha-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 1 / Formula: GLP |
|---|---|
| Molecular weight | Theoretical: 259.151 Da |
| Chemical component information | 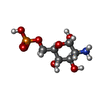 ChemComp-GLP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.9000000000000001 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































