[English] 日本語
 Yorodumi
Yorodumi- EMDB-61413: Cryo-EM structure of Histamine-bound Histamine receptor 4 H4R G p... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Histamine-bound Histamine receptor 4 H4R G protein complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / MEMBRANE PROTEIN/IMMUNE SYSTEM / MEMBRANE PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationHistamine receptors / histamine receptor activity / neurotransmitter receptor activity / adenylate cyclase-inhibiting G protein-coupled acetylcholine receptor signaling pathway / regulation of MAPK cascade / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway ...Histamine receptors / histamine receptor activity / neurotransmitter receptor activity / adenylate cyclase-inhibiting G protein-coupled acetylcholine receptor signaling pathway / regulation of MAPK cascade / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / response to prostaglandin E / D2 dopamine receptor binding / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / regulation of mitotic spindle organization / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor binding / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to peptide hormone / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Glucagon-type ligand receptors / GDP binding / Sensory perception of sweet, bitter, and umami (glutamate) taste / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / G alpha (12/13) signalling events / Inactivation, recovery and regulation of the phototransduction cascade / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / positive regulation of cytosolic calcium ion concentration / retina development in camera-type eye / G protein activity / GTPase binding / Ca2+ pathway / fibroblast proliferation / midbody / cell cortex / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / chemical synaptic transmission / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / ciliary basal body / G protein-coupled receptor signaling pathway / inflammatory response / cell division / lysosomal membrane / GTPase activity / dendrite / synapse / centrosome / GTP binding / protein-containing complex binding / nucleolus / magnesium ion binding / Golgi apparatus / signal transduction / extracellular exosome / nucleoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.58 Å | |||||||||
 Authors Authors | Jin S / Zhang H / Jiang Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Acta Pharmacol Sin / Year: 2026 Journal: Acta Pharmacol Sin / Year: 2026Title: Decoding ligand recognition and constitutive activation of histamine H3 and H4 receptors. Authors: San-Shan Jin / Heng Zhang / Jia-Hui Yan / Can-Rong Wu / Xiao-Qing Cai / Kai Wu / Ming-Wei Wang / H Eric Xu / De-Hua Yang / Yi Jiang /  Abstract: Histamine H3 receptor (H3R) and H4 receptor (H4R) are key members of the histamine receptor family, with H3R as a potential target for narcolepsy treatments and H4R as a candidate for next-generation ...Histamine H3 receptor (H3R) and H4 receptor (H4R) are key members of the histamine receptor family, with H3R as a potential target for narcolepsy treatments and H4R as a candidate for next-generation antihistamines for inflammatory and allergic diseases. Although progress has been made in understanding the structure of histamine receptors, the detailed mechanisms of ligand recognition and receptor antagonism for H3R and H4R remain unclear. In this study, using cryo-electron microscopy, we present an inactive structure of H4R bound to a selective antagonist, adriforant, and two Gi-coupled structures of H3R and H4R in complex with histamine. Our structural and mutagenesis analyses provide insights into the selective binding of adriforant to H4R and the recognition of histamine across histamine receptors. Our findings also uncovered distinct antagonistic mechanisms for H3R and H4R and identified the role of aromatic amino acids on extracellular loop 2 in modulating the constitutive activity of H3R and H4R. These findings advance our knowledge of the functional modulation of histamine receptors, providing a foundation for the development of targeted therapeutics for neurological and immune-related disorders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_61413.map.gz emd_61413.map.gz | 85.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-61413-v30.xml emd-61413-v30.xml emd-61413.xml emd-61413.xml | 22.9 KB 22.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_61413_fsc.xml emd_61413_fsc.xml | 9.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_61413.png emd_61413.png | 85.5 KB | ||
| Filedesc metadata |  emd-61413.cif.gz emd-61413.cif.gz | 6.9 KB | ||
| Others |  emd_61413_half_map_1.map.gz emd_61413_half_map_1.map.gz emd_61413_half_map_2.map.gz emd_61413_half_map_2.map.gz | 83.8 MB 83.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-61413 http://ftp.pdbj.org/pub/emdb/structures/EMD-61413 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-61413 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-61413 | HTTPS FTP |
-Related structure data
| Related structure data |  9jedMC  9jeqC  9jg1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_61413.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_61413.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.832 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_61413_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_61413_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Structural basis for ligand recognition and self-activation of hi...
| Entire | Name: Structural basis for ligand recognition and self-activation of histamine H4 receptors |
|---|---|
| Components |
|
-Supramolecule #1: Structural basis for ligand recognition and self-activation of hi...
| Supramolecule | Name: Structural basis for ligand recognition and self-activation of histamine H4 receptors type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.225801 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: CTLSAEDKAA VERSKMIDRN LREDGEKAAR EVKLLLLGAG ESGKNTIVKQ MKIIHEAGYS EEECKQYKAV VYSNTIQSII AIIRAMGRL KIDFGDSARA DDARQLFVLA GAAEEGFMTA ELAGVIKRLW KDSGVQACFN RSREYQLNDS AAYYLNDLDR I AQPNYIPT ...String: CTLSAEDKAA VERSKMIDRN LREDGEKAAR EVKLLLLGAG ESGKNTIVKQ MKIIHEAGYS EEECKQYKAV VYSNTIQSII AIIRAMGRL KIDFGDSARA DDARQLFVLA GAAEEGFMTA ELAGVIKRLW KDSGVQACFN RSREYQLNDS AAYYLNDLDR I AQPNYIPT QQDVLRTRVK TTGIVETHFT FKDLHFKMFD VGAQRSERKK WIHCFEGVTA IIFCVALSDY DLVLAEDEEM NR MHASMKL FDSICNNKWF TDTSIILFLN KKDLFEEKIK KSPLTICYPE YAGSNTYEEA AAYIQCQFED LNKRKDTKEI YTH FTCSTD TKNVQFVFDA VTDVIIKNNL KDCGLF UniProtKB: Guanine nucleotide-binding protein G(i) subunit alpha-1 |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.285734 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW ...String: SELDQLRQEA EQLKNQIRDA RKACADATLS QITNNIDPVG RIQMRTRRTL RGHLAKIYAM HWGTDSRLLV SASQDGKLII WDSYTTNKV HAIPLRSSWV MTCAYAPSGN YVACGGLDNI CSIYNLKTRE GNVRVSRELA GHTGYLSCCR FLDDNQIVTS S GDTTCALW DIETGQQTTT FTGHTGDVMS LSLAPDTRLF VSGACDASAK LWDVREGMCR QTFTGHESDI NAICFFPNGN AF ATGSDDA TCRLFDLRAD QELMTYSHDN IICGITSVSF SKSGRLLLAG YDDFNCNVWD ALKADRAGVL AGHDNRVSCL GVT DDGMAV ATGSWDSFLK IWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.375332 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: NTASIAQARK LVEQLKMEAN IDRIKVSKAA ADLMAYCEAH AKEDPLLTPV PASENPFR UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Histamine H4 receptor
| Macromolecule | Name: Histamine H4 receptor / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 44.543766 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MPDTNSTINL SLSTRVTLAF FMSLVAFAIM LGNALVILAF VVDKNLRHRS SYFFLNLAIS DFFVGVISIP LYIPHTLFEW DFGKEICVF WLTTDYLLCT ASVYNIVLIS YDRYLSVSNA VSYRTQHTGV LKIVTLMVAV WVLAFLVNGP MILVSESWKD E GSECEPGF ...String: MPDTNSTINL SLSTRVTLAF FMSLVAFAIM LGNALVILAF VVDKNLRHRS SYFFLNLAIS DFFVGVISIP LYIPHTLFEW DFGKEICVF WLTTDYLLCT ASVYNIVLIS YDRYLSVSNA VSYRTQHTGV LKIVTLMVAV WVLAFLVNGP MILVSESWKD E GSECEPGF FSEWYILAIT SFLEFVIPVI LVAYFNMNIY WSLWKRDHLS RCQSHPGLTA VSSNICGHSF RGRLSSRRSL SA STEVPAS FHSERQRRKS SLMFSSRTKM NSNTIASKMG SFSQSDSVAL HQREHVELLR ARRLAKSLAI LLGVFAVCWA PYS LFTIVL SFYSSATGPK SVWYRIAFWL QWFNSFVNPL LYPLCHKRFQ KAFLKIFCIK KQPLPSQHSR SVSS UniProtKB: Histamine H4 receptor |
-Macromolecule #5: ScFv16
| Macromolecule | Name: ScFv16 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 26.277299 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: VQLVESGGGL VQPGGSRKLS CSASGFAFSS FGMHWVRQAP EKGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSED TAMYYCVRSI YYYGSSPFDF WGQGTTLTVS AGGGGSGGGG SGGGGSADIV MTQATSSVPV TPGESVSISC R SSKSLLHS ...String: VQLVESGGGL VQPGGSRKLS CSASGFAFSS FGMHWVRQAP EKGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSED TAMYYCVRSI YYYGSSPFDF WGQGTTLTVS AGGGGSGGGG SGGGGSADIV MTQATSSVPV TPGESVSISC R SSKSLLHS NGNTYLYWFL QRPGQSPQLL IYRMSNLASG VPDRFSGSGS GTAFTLTISR LEAEDVGVYY CMQHLEYPLT FG AGTKLEL |
-Macromolecule #6: HISTAMINE
| Macromolecule | Name: HISTAMINE / type: ligand / ID: 6 / Number of copies: 1 / Formula: HSM |
|---|---|
| Molecular weight | Theoretical: 111.145 Da |
| Chemical component information | 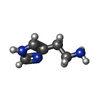 ChemComp-HSM: |
-Macromolecule #7: PHOSPHATE ION
| Macromolecule | Name: PHOSPHATE ION / type: ligand / ID: 7 / Number of copies: 1 / Formula: PO4 |
|---|---|
| Molecular weight | Theoretical: 94.971 Da |
| Chemical component information |  ChemComp-PO4: |
-Macromolecule #8: water
| Macromolecule | Name: water / type: ligand / ID: 8 / Number of copies: 3 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: DIFFRACTION / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





































