[English] 日本語
 Yorodumi
Yorodumi- EMDB-61201: Arabidopsis high-affinity urea transport DUR3 in the urea-bound o... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Arabidopsis high-affinity urea transport DUR3 in the urea-bound occluded conformation, dimeric state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DUR3 / high-affinity urea transporter / urea transporter / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationurea transmembrane transporter activity / urea transmembrane transport / symporter activity / cellular response to nitrogen starvation / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | An W / Gao Y / Zhang XC | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Structural basis of urea transport by Arabidopsis thaliana DUR3. Authors: Weidong An / Yiwei Gao / Laihua Liu / Qinru Bai / Jun Zhao / Yan Zhao / Xuejun C Zhang /  Abstract: Urea is a primary nitrogen source used as fertilizer in agricultural plant production and a crucial nitrogen metabolite in plants, playing an essential role in modern agriculture. In plants, DUR3 is ...Urea is a primary nitrogen source used as fertilizer in agricultural plant production and a crucial nitrogen metabolite in plants, playing an essential role in modern agriculture. In plants, DUR3 is a proton-driven high-affinity urea transporter located on the plasma membrane. It not only absorbs external low-concentration urea as a nutrient but also facilitates nitrogen transfer by recovering urea from senescent leaves. Despite its importance, the high-affinity urea transport mechanism in plants remains insufficiently understood. In this study, we determine the structures of Arabidopsis thaliana DUR3 in two different conformations: the inward-facing open state of the apo structure and the occluded urea-bound state, with overall resolutions of 2.8 Å and 3.0 Å, respectively. By comparing these structures and analyzing their functional characteristics, we elucidated how urea molecules are specifically recognized. In the urea-bound structure, we identified key titratable amino acid residues and proposed a model for proton involvement in urea transport based on structural and functional data. This study enhances our understanding of proton-driven urea transport mechanisms in DUR3. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_61201.map.gz emd_61201.map.gz | 198.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-61201-v30.xml emd-61201-v30.xml emd-61201.xml emd-61201.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_61201.png emd_61201.png | 120.8 KB | ||
| Filedesc metadata |  emd-61201.cif.gz emd-61201.cif.gz | 6 KB | ||
| Others |  emd_61201_half_map_1.map.gz emd_61201_half_map_1.map.gz emd_61201_half_map_2.map.gz emd_61201_half_map_2.map.gz | 195 MB 195 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-61201 http://ftp.pdbj.org/pub/emdb/structures/EMD-61201 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-61201 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-61201 | HTTPS FTP |
-Validation report
| Summary document |  emd_61201_validation.pdf.gz emd_61201_validation.pdf.gz | 891.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_61201_full_validation.pdf.gz emd_61201_full_validation.pdf.gz | 891.4 KB | Display | |
| Data in XML |  emd_61201_validation.xml.gz emd_61201_validation.xml.gz | 15.5 KB | Display | |
| Data in CIF |  emd_61201_validation.cif.gz emd_61201_validation.cif.gz | 18.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-61201 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-61201 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-61201 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-61201 | HTTPS FTP |
-Related structure data
| Related structure data |  9j7cMC  9j7dC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_61201.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_61201.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_61201_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_61201_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Arabidopsis high-affinity urea transporter
| Entire | Name: Arabidopsis high-affinity urea transporter |
|---|---|
| Components |
|
-Supramolecule #1: Arabidopsis high-affinity urea transporter
| Supramolecule | Name: Arabidopsis high-affinity urea transporter / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Urea-proton symporter DUR3
| Macromolecule | Name: Urea-proton symporter DUR3 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 75.977445 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MATCPPFDFS TKYYDGDGGC QRQSSFFGGT TVLDQGVGYA VILGFGAFFA VFTSFLVWLE KRYVGARHTS EWFNTAGRNV KTGLIASVI VSQWTWAATI LQSSNVAWQY GVSGPFWYAS GATIQVLLFG VMAIEIKRKA PNAHTVCEIV KARWGTATHI V FLVFCLAT ...String: MATCPPFDFS TKYYDGDGGC QRQSSFFGGT TVLDQGVGYA VILGFGAFFA VFTSFLVWLE KRYVGARHTS EWFNTAGRNV KTGLIASVI VSQWTWAATI LQSSNVAWQY GVSGPFWYAS GATIQVLLFG VMAIEIKRKA PNAHTVCEIV KARWGTATHI V FLVFCLAT NVVVTAMLLL GGSAVVNALT GVNLYAASFL IPLGVVVYTL AGGLKATFLA SYVHSVIVHV ALVVFVFLVY TS SKELGSP SVVYDRLKDM VAKSRSCTEP LSHHGQACGP VDGNFRGSYL TMLSSGGAVF GLINIVGNFG TVFVDNGYWV SAI AARPSS THKGYLLGGL VWFAVPFSLA TSLGLGALAL DLPISKDEAD RGLVPPATAI ALMGKSGSLL LLTMLFMAVT SAGS SELIA VSSLFTYDIY RTYINPRATG RQILKISRCA VLGFGCFMGI LAVVLNKAGV SLGWMYLAMG VLIGSAVIPI AFMLL WSKA NAFGAILGAT SGCVFGIITW LTTAKTQYGR VDLDSTGKNG PMLAGNLVAI LTGGLIHAVC SLVRPQNYDW STTREI KVV EAYASGDEDV DVPAEELREE KLRRAKAWIV KWGLVFTILI VVIWPVLSLP ARVFSRGYFW FWAIVAIAWG TIGSIVI IG LPLVESWDTI KSVCMGMFTN DRVMKKLDDL NHRLRALTMA VPEAEKIYLL ELEKTKKNDE EG UniProtKB: Urea-proton symporter DUR3 |
-Macromolecule #2: CHOLESTEROL HEMISUCCINATE
| Macromolecule | Name: CHOLESTEROL HEMISUCCINATE / type: ligand / ID: 2 / Number of copies: 4 / Formula: Y01 |
|---|---|
| Molecular weight | Theoretical: 486.726 Da |
| Chemical component information |  ChemComp-Y01: |
-Macromolecule #3: UREA
| Macromolecule | Name: UREA / type: ligand / ID: 3 / Number of copies: 2 / Formula: URE |
|---|---|
| Molecular weight | Theoretical: 60.055 Da |
| Chemical component information | 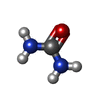 ChemComp-URE: |
-Macromolecule #4: HEXADECANE
| Macromolecule | Name: HEXADECANE / type: ligand / ID: 4 / Number of copies: 2 / Formula: R16 |
|---|---|
| Molecular weight | Theoretical: 226.441 Da |
| Chemical component information |  ChemComp-R16: |
-Macromolecule #5: TETRADECANE
| Macromolecule | Name: TETRADECANE / type: ligand / ID: 5 / Number of copies: 1 / Formula: C14 |
|---|---|
| Molecular weight | Theoretical: 198.388 Da |
| Chemical component information |  ChemComp-C14: |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 6 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































