[English] 日本語
 Yorodumi
Yorodumi- EMDB-61140: CryoEM structure of human XPR1 in complex with phosphate in state B -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of human XPR1 in complex with phosphate in state B | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Phosphate / Transport / XPR1 / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationphosphate transmembrane transporter activity / phosphate ion transport / intracellular phosphate ion homeostasis / cellular response to phosphate starvation / phosphate ion transmembrane transport / inositol hexakisphosphate binding / efflux transmembrane transporter activity / response to virus / virus receptor activity / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Zhang WH / Chen YK / Guan ZY / Liu Z | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Structural insights into the mechanism of phosphate recognition and transport by XPR1. Authors: Wenhui Zhang / Yanke Chen / Zeyuan Guan / Yong Wang / Meng Tang / Zhangmeng Du / Jie Zhang / Meng Cheng / Jiaqi Zuo / Yan Liu / Qiang Wang / Yanjun Liu / Delin Zhang / Ping Yin / Ling Ma / Zhu Liu /  Abstract: XPR1 is the sole protein known to transport inorganic phosphate (Pi) out of cells, a function conserved across species from yeast to mammals. Human XPR1 variants lead to cerebral calcium-phosphate ...XPR1 is the sole protein known to transport inorganic phosphate (Pi) out of cells, a function conserved across species from yeast to mammals. Human XPR1 variants lead to cerebral calcium-phosphate deposition and primary familial brain calcification (PFBC), a hereditary neurodegenerative disorder. Here, we present the cryo-EM structure of human XPR1 in both its Pi-unbound and various Pi-bound states. XPR1 features 10 transmembrane α-helices forming an ion channel-like structure, with multiple Pi recognition sites along the channel. Pathogenic mutations in two arginine residues, which line the translocation channel, disrupt Pi transport. Molecular dynamics simulations reveal that Pi ion undergoes a stepwise transition through the sequential recognition sites during the transport process. Together with functional analyses, our results suggest that this sequential arrangement allows XPR1 to facilitate Pi ion passage via a "relay" process, and they establish a framework for the interpretation of disease-related mutations and for the development of future therapeutics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_61140.map.gz emd_61140.map.gz | 78.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-61140-v30.xml emd-61140-v30.xml emd-61140.xml emd-61140.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_61140.png emd_61140.png | 56.9 KB | ||
| Filedesc metadata |  emd-61140.cif.gz emd-61140.cif.gz | 6.1 KB | ||
| Others |  emd_61140_half_map_1.map.gz emd_61140_half_map_1.map.gz emd_61140_half_map_2.map.gz emd_61140_half_map_2.map.gz | 77.7 MB 77.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-61140 http://ftp.pdbj.org/pub/emdb/structures/EMD-61140 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-61140 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-61140 | HTTPS FTP |
-Related structure data
| Related structure data |  9j52MC  9j4xC  9j51C  9j53C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_61140.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_61140.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
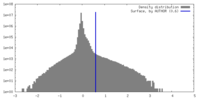
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_61140_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_61140_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human XPR1
| Entire | Name: Human XPR1 |
|---|---|
| Components |
|
-Supramolecule #1: Human XPR1
| Supramolecule | Name: Human XPR1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Solute carrier family 53 member 1
| Macromolecule | Name: Solute carrier family 53 member 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 84.595844 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKFAEHLSAH ITPEWRKQYI QYEAFKDMLY SAQDQAPSVE VTDEDTVKRY FAKFEEKFFQ TCEKELAKIN TFYSEKLAEA QRRFATLQN ELQSSLDAQK ESTGVTTLRQ RRKPVFHLSH EERVQHRNIK DLKLAFSEFY LSLILLQNYQ NLNFTGFRKI L KKHDKILE ...String: MKFAEHLSAH ITPEWRKQYI QYEAFKDMLY SAQDQAPSVE VTDEDTVKRY FAKFEEKFFQ TCEKELAKIN TFYSEKLAEA QRRFATLQN ELQSSLDAQK ESTGVTTLRQ RRKPVFHLSH EERVQHRNIK DLKLAFSEFY LSLILLQNYQ NLNFTGFRKI L KKHDKILE TSRGADWRVA HVEVAPFYTC KKINQLISET EAVVTNELED GDRQKAMKRL RVPPLGAAQP APAWTTFRVG LF CGIFIVL NITLVLAAVF KLETDRSIWP LIRIYRGGFL LIEFLFLLGI NTYGWRQAGV NHVLIFELNP RSNLSHQHLF EIA GFLGIL WCLSLLACFF APISVIPTYV YPLALYGFMV FFLINPTKTF YYKSRFWLLK LLFRVFTAPF HKVGFADFWL ADQL NSLSV ILMDLEYMIC FYSLELKWDE SKGLLPNNSE ESGICHKYTY GVRAIVQCIP AWLRFIQCLR RYRDTKRAFP HLVNA GKYS TTFFMVTFAA LYSTHKERGH SDTMVFFYLW IVFYIISSCY TLIWDLKMDW GLFDKNAGEN TFLREEIVYP QKAYYY CAI IEDVILRFAW TIQISITSTT LLPHSGDIIA TVFAPLEVFR RFVWNFFRLE NEHLNNCGEF RAVRDISVAP LNADDQT LL EQMMDQDDGV RNRQKNRSWK YNQSISLRRP RLASQSKARD TKVLIEDTDD EANTLEDYKD HDGDYKDHDI DYKDDDDK UniProtKB: Solute carrier family 53 member 1 |
-Macromolecule #2: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 2 / Number of copies: 4 / Formula: PC1 |
|---|---|
| Molecular weight | Theoretical: 790.145 Da |
| Chemical component information |  ChemComp-PC1: |
-Macromolecule #3: PHOSPHATE ION
| Macromolecule | Name: PHOSPHATE ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: PO4 |
|---|---|
| Molecular weight | Theoretical: 94.971 Da |
| Chemical component information |  ChemComp-PO4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.7 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)