[English] 日本語
 Yorodumi
Yorodumi- EMDB-6101: CryoEM reveals different coronin binding modes for ADP- and ADP-B... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6101 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM reveals different coronin binding modes for ADP- and ADP-BeFx- actin filaments | |||||||||
 Map data Map data | Actin-coronin complex in ADP-BeFx state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | actin / coronin | |||||||||
| Function / homology |  Function and homology information Function and homology informationactin filament debranching / positive regulation of Arp2/3 complex-mediated actin nucleation / actin cortical patch localization / Arp2/3 complex binding / negative regulation of Arp2/3 complex-mediated actin nucleation / actin cortical patch / cytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / troponin I binding ...actin filament debranching / positive regulation of Arp2/3 complex-mediated actin nucleation / actin cortical patch localization / Arp2/3 complex binding / negative regulation of Arp2/3 complex-mediated actin nucleation / actin cortical patch / cytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / troponin I binding / filamentous actin / mesenchyme migration / actin filament bundle / actin filament bundle assembly / skeletal muscle myofibril / striated muscle thin filament / skeletal muscle thin filament assembly / actin monomer binding / microtubule-based process / stress fiber / skeletal muscle fiber development / titin binding / actin filament polymerization / actin filament organization / filopodium / actin filament / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / calcium-dependent protein binding / actin filament binding / lamellipodium / cell body / microtubule binding / protein-macromolecule adaptor activity / hydrolase activity / protein domain specific binding / calcium ion binding / positive regulation of gene expression / magnesium ion binding / ATP binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 8.6 Å | |||||||||
 Authors Authors | Ge P / Durer ZAO / Kudryashov D / Zhou ZH / Reisler E | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2014 Journal: Nat Struct Mol Biol / Year: 2014Title: Cryo-EM reveals different coronin binding modes for ADP- and ADP-BeFx actin filaments. Authors: Peng Ge / Zeynep A Oztug Durer / Dmitri Kudryashov / Z Hong Zhou / Emil Reisler /  Abstract: Essential cellular processes involving the actin cytoskeleton are regulated by auxiliary proteins that can sense the nucleotide state of actin. Here we report cryo-EM structures for ADP-bound and ADP- ...Essential cellular processes involving the actin cytoskeleton are regulated by auxiliary proteins that can sense the nucleotide state of actin. Here we report cryo-EM structures for ADP-bound and ADP-beryllium fluoride (ADP-BeFx, an ADP-Pi mimic)-bound actin filaments in complex with the β-propeller domain of yeast coronin 1 (crn1), at 8.6-Å resolution. Our structures reveal the main differences in the interaction of coronin with the two nucleotide states of F-actin. We derived pseudoatomic models by fitting the atomic structures of actin and coronin into the EM envelopes and confirmed the identified interfaces on actin by chemical cross-linking, fluorescence spectroscopy and actin mutagenesis. The models offer a structural explanation for the nucleotide-dependent effects of coronin on cofilin-assisted remodeling of F-actin. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6101.map.gz emd_6101.map.gz | 118.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6101-v30.xml emd-6101-v30.xml emd-6101.xml emd-6101.xml | 10.7 KB 10.7 KB | Display Display |  EMDB header EMDB header |
| Images |  400_6101.gif 400_6101.gif 80_6101.gif 80_6101.gif | 33.7 KB 2.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6101 http://ftp.pdbj.org/pub/emdb/structures/EMD-6101 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6101 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6101 | HTTPS FTP |
-Validation report
| Summary document |  emd_6101_validation.pdf.gz emd_6101_validation.pdf.gz | 79.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_6101_full_validation.pdf.gz emd_6101_full_validation.pdf.gz | 78.3 KB | Display | |
| Data in XML |  emd_6101_validation.xml.gz emd_6101_validation.xml.gz | 493 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6101 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6101 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6101 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6101 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6101.map.gz / Format: CCP4 / Size: 122.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6101.map.gz / Format: CCP4 / Size: 122.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Actin-coronin complex in ADP-BeFx state | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.437 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
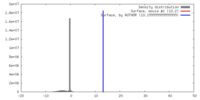
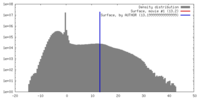
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Actin-Coronin complex in ADP-BeFx state
| Entire | Name: Actin-Coronin complex in ADP-BeFx state |
|---|---|
| Components |
|
-Supramolecule #1000: Actin-Coronin complex in ADP-BeFx state
| Supramolecule | Name: Actin-Coronin complex in ADP-BeFx state / type: sample / ID: 1000 Oligomeric state: helix; one coronin subunit per one actin subunit Number unique components: 2 |
|---|
-Macromolecule #1: actin
| Macromolecule | Name: actin / type: protein_or_peptide / ID: 1 / Oligomeric state: helix / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42 KDa |
| Sequence | UniProtKB: Actin, alpha skeletal muscle |
-Macromolecule #2: Coronin 1
| Macromolecule | Name: Coronin 1 / type: protein_or_peptide / ID: 2 / Name.synonym: crn1 / Oligomeric state: helix / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 72 KDa |
| Recombinant expression | Organism:  |
| Sequence | UniProtKB: Coronin-like protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Details: 10 mM Tris, 0.2 mM CaCl2, 1 mM MgCl2, 50 mM KCl, 0.1 mM BeCl2, 5 mM NaF, 1 mM DTT |
|---|---|
| Grid | Details: 300 Me Quantifoil R1.2/1.3 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 77 K / Instrument: FEI VITROBOT MARK IV Method: blot force 1, blot time 4s, blot once, wait time 2s, drain time 2s; sample volume applied to each grid was 2.5 uL |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Average: 77 K |
| Alignment procedure | Legacy - Astigmatism: Relion software correction |
| Date | Dec 12, 2012 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Digitization - Sampling interval: 15 µm / Number real images: 1200 / Average electron dose: 25 e/Å2 / Bits/pixel: 16 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 104384 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 2.0 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Relion-based IHRSR |
|---|---|
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 28.23 Å Applied symmetry - Helical parameters - Δ&Phi: 166.3 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 8.6 Å / Resolution method: OTHER / Software - Name: Relion, IHRSR |
| CTF correction | Details: Each particle |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)