+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-5671 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cross-Neutralizing Human Anti-Poliovirus Antibodies Bind the Recognition Site for Cellular Receptor | |||||||||
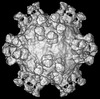
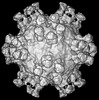
 マップデータ マップデータ | Cryo-EM reconstruction of type 2 polio virus with A12 Fab attached | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Polio / Type 2 Polio / A12 | |||||||||
| 生物種 |  Human poliovirus 2 (ポリオウイルス) Human poliovirus 2 (ポリオウイルス) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 20.0 Å | |||||||||
 データ登録者 データ登録者 | Chen Z / Fischer ER / Kouiavskaia D / Hansen BT / Ludtke SJ / Bidzhieva B / Makiya M / Purcell RH / Chumakov K | |||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2013 ジャーナル: Proc Natl Acad Sci U S A / 年: 2013タイトル: Cross-neutralizing human anti-poliovirus antibodies bind the recognition site for cellular receptor. 著者: Zhaochun Chen / Elizabeth R Fischer / Diana Kouiavskaia / Bryan T Hansen / Steven J Ludtke / Bella Bidzhieva / Michelle Makiya / Liane Agulto / Robert H Purcell / Konstantin Chumakov /  要旨: Most structural information about poliovirus interaction with neutralizing antibodies was obtained in the 1980s in studies of mouse monoclonal antibodies. Recently we have isolated a number of ...Most structural information about poliovirus interaction with neutralizing antibodies was obtained in the 1980s in studies of mouse monoclonal antibodies. Recently we have isolated a number of human/chimpanzee anti-poliovirus antibodies and demonstrated that one of them, MAb A12, could neutralize polioviruses of both serotypes 1 and 2. This communication presents data on isolation of an additional cross-neutralizing antibody (F12) and identification of a previously unknown epitope on the surface of poliovirus virions. Epitope mapping was performed by sequencing of antibody-resistant mutants and by cryo-EM of complexes of virions with Fab fragments. The results have demonstrated that both cross-neutralizing antibodies bind the site located at the bottom of the canyon surrounding the fivefold axis of symmetry that was previously shown to interact with cellular poliovirus receptor CD155. However, the same antibody binds to serotypes 1 and 2 through different specific interactions. It was also shown to interact with type 3 poliovirus, albeit with about 10-fold lower affinity, insufficient for effective neutralization. Antibody interaction with the binding site of the cellular receptor may explain its broad reactivity and suggest that further screening or antibody engineering could lead to a universal antibody capable of neutralizing all three serotypes of poliovirus. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_5671.map.gz emd_5671.map.gz | 11.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-5671-v30.xml emd-5671-v30.xml emd-5671.xml emd-5671.xml | 10.7 KB 10.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_5671_1.jpg emd_5671_1.jpg | 150.8 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5671 http://ftp.pdbj.org/pub/emdb/structures/EMD-5671 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5671 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5671 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_5671_validation.pdf.gz emd_5671_validation.pdf.gz | 78.5 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_5671_full_validation.pdf.gz emd_5671_full_validation.pdf.gz | 77.6 KB | 表示 | |
| XML形式データ |  emd_5671_validation.xml.gz emd_5671_validation.xml.gz | 494 B | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5671 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5671 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5671 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5671 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_5671.map.gz / 形式: CCP4 / 大きさ: 50.4 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_5671.map.gz / 形式: CCP4 / 大きさ: 50.4 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Cryo-EM reconstruction of type 2 polio virus with A12 Fab attached | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 これらの図は立方格子座標系で作成されたものです | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.8 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Fab Fragment of A12 antibody bound to Type 2 poliovirus
| 全体 | 名称: Fab Fragment of A12 antibody bound to Type 2 poliovirus |
|---|---|
| 要素 |
|
-超分子 #1000: Fab Fragment of A12 antibody bound to Type 2 poliovirus
| 超分子 | 名称: Fab Fragment of A12 antibody bound to Type 2 poliovirus タイプ: sample / ID: 1000 / Number unique components: 2 |
|---|
-超分子 #1: Human poliovirus 2
| 超分子 | 名称: Human poliovirus 2 / タイプ: virus / ID: 1 / NCBI-ID: 12083 / 生物種: Human poliovirus 2 / データベース: NCBI / ウイルスタイプ: VIRION / ウイルス・単離状態: SPECIES / ウイルス・エンベロープ: Yes / ウイルス・中空状態: No |
|---|---|
| 宿主 | 生物種:  Homo sapiens (ヒト) / 別称: VERTEBRATES Homo sapiens (ヒト) / 別称: VERTEBRATES |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 1 mg/mL |
|---|---|
| グリッド | 詳細: 4 uL aliquots of sample were applied to freshly glow-discharged 200 mesh r2/2 Quantifoil copper grids suspended by forceps in the FEI Mark IV Vitrobot. |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 80 % / チャンバー内温度: 65 K / 装置: FEI VITROBOT MARK IV 手法: Specimens were vitrified, after blotting for 2 seconds with a blot force of 5 at 80% relative humidity, by plunge freezing into liquid ethane. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 温度 | 最低: 64 K / 最高: 66 K / 平均: 65 K |
| 日付 | 2011年3月3日 |
| 撮影 | カテゴリ: CCD / フィルム・検出器のモデル: GENERIC CCD / デジタル化 - サンプリング間隔: 2 µm / 実像数: 150 / 平均電子線量: 15 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 80173 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm / 最大 デフォーカス(公称値): 5.0 µm / 最小 デフォーカス(公称値): 1.0 µm / 倍率(公称値): 47000 |
| 試料ステージ | 試料ホルダー: Liquid Nitrogen cooled 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 詳細 | Particles with a defocus range of 1-5 um were boxed with EMAN2, and then processed using the standard single particle reconstruction procedure with full CTF correction. |
|---|---|
| CTF補正 | 詳細: each image |
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 20.0 Å / 解像度の算出法: FSC 0.5 CUT-OFF / ソフトウェア - 名称: EMAN2 / 使用した粒子像数: 1500 |
| 最終 2次元分類 | クラス数: 10 |
-原子モデル構築 1
| 初期モデル | PDB ID: Chain - #0 - Chain ID: A / Chain - #1 - Chain ID: B / Chain - #2 - Chain ID: C / Chain - #3 - Chain ID: D |
|---|---|
| ソフトウェア | 名称:  Chimera Chimera |
| 精密化 | 空間: REAL / プロトコル: RIGID BODY FIT |
 ムービー
ムービー コントローラー
コントローラー










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)