+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5525 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Electron cryo-microscopy of ABC BmrA in 12-fold symmetry rings | |||||||||
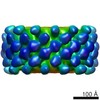
 Map data Map data | Reconstruction of BmrA in a lipidic environment. Reconstruction is D12 symmetrized. 24 homodimers of BmrA are inserted into a lipid bilayer. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC transporter / exporter / bmra / yvcc / transmembrane protein / reconstitution into lipids / membrane protein | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 23.0 Å | |||||||||
 Authors Authors | Fribourg PF / Chami M / Sorzano CO / Gubellini F / Marabini R / Marco S / Jault JM / Levy D | |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2014 Journal: J Mol Biol / Year: 2014Title: 3D cryo-electron reconstruction of BmrA, a bacterial multidrug ABC transporter in an inward-facing conformation and in a lipidic environment. Authors: Pierre Frederic Fribourg / Mohamed Chami / Carlos Oscar S Sorzano / Francesca Gubellini / Roberto Marabini / Sergio Marco / Jean-Michel Jault / Daniel Lévy /    Abstract: ABC (ATP-binding cassette) membrane exporters are efflux transporters of a wide diversity of molecule across the membrane at the expense of ATP. A key issue regarding their catalytic cycle is whether ...ABC (ATP-binding cassette) membrane exporters are efflux transporters of a wide diversity of molecule across the membrane at the expense of ATP. A key issue regarding their catalytic cycle is whether or not their nucleotide-binding domains (NBDs) are physically disengaged in the resting state. To settle this controversy, we obtained structural data on BmrA, a bacterial multidrug homodimeric ABC transporter, in a membrane-embedded state. BmrA in the apostate was reconstituted in lipid bilayers forming a mixture of ring-shaped structures of 24 or 39 homodimers. Three-dimensional models of the ring-shaped structures of 24 or 39 homodimers were calculated at 2.3 nm and 2.5 nm resolution from cryo-electron microscopy, respectively. In these structures, BmrA adopts an inward-facing open conformation similar to that found in mouse P-glycoprotein structure with the NBDs separated by 3 nm. Both lipidic leaflets delimiting the transmembrane domains of BmrA were clearly resolved. In planar membrane sheets, the NBDs were even more separated. BmrA in an ATP-bound conformation was determined from two-dimensional crystals grown in the presence of ATP and vanadate. A projection map calculated at 1.6 nm resolution shows an open outward-facing conformation. Overall, the data are consistent with a mechanism of drug transport involving large conformational changes of BmrA and show that a bacterial ABC exporter can adopt at least two open inward conformations in lipid membrane. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5525.map.gz emd_5525.map.gz | 8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5525-v30.xml emd-5525-v30.xml emd-5525.xml emd-5525.xml | 10.9 KB 10.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5525.tif emd_5525.tif | 732.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5525 http://ftp.pdbj.org/pub/emdb/structures/EMD-5525 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5525 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5525 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5525.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5525.map.gz / Format: CCP4 / Size: 29.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of BmrA in a lipidic environment. Reconstruction is D12 symmetrized. 24 homodimers of BmrA are inserted into a lipid bilayer. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.5 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : BmrA reconstituted at high density into lipid bilayer.
| Entire | Name: BmrA reconstituted at high density into lipid bilayer. |
|---|---|
| Components |
|
-Supramolecule #1000: BmrA reconstituted at high density into lipid bilayer.
| Supramolecule | Name: BmrA reconstituted at high density into lipid bilayer. type: sample / ID: 1000 Oligomeric state: 24 Homodimers of BmrA inserted into lipid bilayer Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 3.12 MDa / Theoretical: 3.12 MDa |
-Macromolecule #1: BmrA
| Macromolecule | Name: BmrA / type: protein_or_peptide / ID: 1 / Name.synonym: YvcC / Details: 24 homodimers of inserted into lipid bilayer / Number of copies: 24 / Oligomeric state: Dimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 3.12 MDa / Theoretical: 3.12 MDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 7.8 / Details: 50mM HEPES, 100mM NaCl |
| Grid | Details: Ted Pella inc. holey formvar grid |
| Vitrification | Cryogen name: ETHANE / Instrument: HOMEMADE PLUNGER Details: specimen linked to a Ni++-NTA-DOGS/DOPC lipid monolayer Method: Blot for 3 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Date | Jan 1, 2003 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: NIKON COOLSCAN / Number real images: 102 / Average electron dose: 15 e/Å2 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 50000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.26 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 2.6 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | Xmipp CL2D classification and Projection matching refinement with Invert Fourier 3D reconstruction |
|---|---|
| CTF correction | Details: Micrograph based |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 23.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: Xmipp / Number images used: 1188 |
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Protocol: Rigid body |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Cross correlation factor |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















