[English] 日本語
 Yorodumi
Yorodumi- EMDB-5430: dATP-inhibited class Ia ribonucleotide reductase from E. coli: al... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5430 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
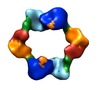
| Title | dATP-inhibited class Ia ribonucleotide reductase from E. coli: alpha4beta4 closed ring conformation | |||||||||
 Map data Map data | closed alpha4beta4 ring | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ribonucleotide reductase / allostery / inhibition / nucleotide metabolism | |||||||||
| Function / homology | ribonucleoside-diphosphate reductase complex Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 23.4 Å | |||||||||
 Authors Authors | Zimanyi CM / Ando N / Brignole E / Asturias FJ / Stubbe J / Drennan CL | |||||||||
 Citation Citation |  Journal: Structure / Year: 2012 Journal: Structure / Year: 2012Title: Tangled up in knots: structures of inactivated forms of E. coli class Ia ribonucleotide reductase. Authors: Christina M Zimanyi / Nozomi Ando / Edward J Brignole / Francisco J Asturias / Joanne Stubbe / Catherine L Drennan /  Abstract: Ribonucleotide reductases (RNRs) provide the precursors for DNA biosynthesis and repair and are successful targets for anticancer drugs such as clofarabine and gemcitabine. Recently, we reported that ...Ribonucleotide reductases (RNRs) provide the precursors for DNA biosynthesis and repair and are successful targets for anticancer drugs such as clofarabine and gemcitabine. Recently, we reported that dATP inhibits E. coli class Ia RNR by driving formation of RNR subunits into α4β4 rings. Here, we present the first X-ray structure of a gemcitabine-inhibited E. coli RNR and show that the previously described α4β4 rings can interlock to form an unprecedented (α4β4)2 megacomplex. This complex is also seen in a higher-resolution dATP-inhibited RNR structure presented here, which employs a distinct crystal lattice from that observed in the gemcitabine-inhibited case. With few reported examples of protein catenanes, we use data from small-angle X-ray scattering and electron microscopy to both understand the solution conditions that contribute to concatenation in RNRs as well as present a mechanism for the formation of these unusual structures. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5430.map.gz emd_5430.map.gz | 1.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5430-v30.xml emd-5430-v30.xml emd-5430.xml emd-5430.xml | 11.7 KB 11.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5430_1.png emd_5430_1.png | 91.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5430 http://ftp.pdbj.org/pub/emdb/structures/EMD-5430 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5430 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5430 | HTTPS FTP |
-Validation report
| Summary document |  emd_5430_validation.pdf.gz emd_5430_validation.pdf.gz | 78.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5430_full_validation.pdf.gz emd_5430_full_validation.pdf.gz | 77.6 KB | Display | |
| Data in XML |  emd_5430_validation.xml.gz emd_5430_validation.xml.gz | 493 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5430 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5430 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5430 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5430 | HTTPS FTP |
-Related structure data
| Related structure data |  5431C  5432C  5433C  5434C  5435C  5436C  5437C  4ermC  4erpC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5430.map.gz / Format: CCP4 / Size: 1.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5430.map.gz / Format: CCP4 / Size: 1.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | closed alpha4beta4 ring | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X: 4.36 Å / Y: 4.36 Å / Z: 6.54 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
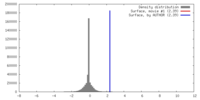
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : E. coli Class Ia ribonucleotide reductase
| Entire | Name: E. coli Class Ia ribonucleotide reductase |
|---|---|
| Components |
|
-Supramolecule #1000: E. coli Class Ia ribonucleotide reductase
| Supramolecule | Name: E. coli Class Ia ribonucleotide reductase / type: sample / ID: 1000 Oligomeric state: two alpha2 subunits in complex with two beta2 subunits Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 517 KDa / Theoretical: 517 KDa / Method: Calculated from amino acid sequence of subunits |
-Macromolecule #1: E. coli Class Ia ribonucleotide reductase
| Macromolecule | Name: E. coli Class Ia ribonucleotide reductase / type: protein_or_peptide / ID: 1 / Name.synonym: RNR / Details: closed alpha4beta4 ring / Number of copies: 1 / Oligomeric state: hetero-octamer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 517 KDa / Theoretical: 517 KDa |
| Recombinant expression | Organism:  |
| Sequence | GO: ribonucleoside-diphosphate reductase complex |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 Details: 50 mM HEPES, 15 mM MgCl2, 1 mM EDTA, 1 mM CDP, 0.05 mM dATP |
|---|---|
| Staining | Type: NEGATIVE Details: 5ul protein, washed immediately 3x [2% uranyl acetate, 0.2% trehalose], carbon sandwich |
| Grid | Details: thin carbon support on 300 mesh Cu/Rh grid, glow discharge in amylamine |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Date | Jul 31, 2010 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Number real images: 89 / Average electron dose: 25 e/Å2 / Details: images acquired as tilt-pairs / Bits/pixel: 16 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 1.801 µm / Nominal defocus min: 0.275 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: room temperature / Specimen holder model: SIDE ENTRY, EUCENTRIC / Tilt angle min: -59.1 / Tilt angle max: -54.3 |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Semi-automated particle selection from untilted images with EMAN2. Particles matched in tilted images using modified TiltPicker. Processed in SPIDER. |
|---|---|
| CTF correction | Details: each particle |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 23.4 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: SPIDER / Number images used: 1039 |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - #0 - Chain ID: A / Chain - #1 - Chain ID: B / Chain - #2 - Chain ID: D / Chain - #3 - Chain ID: E |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Protocol: rigid body. Subunits iteratively fit with Chimera |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: correlation |
-Atomic model buiding 2
| Initial model | PDB ID: Chain - #0 - Chain ID: A / Chain - #1 - Chain ID: B |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Protocol: rigid body. Subunits iteratively fit with Chimera |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: correlation |
 Movie
Movie Controller
Controller


 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)