[English] 日本語
 Yorodumi
Yorodumi- EMDB-5417: Cryo-electron microscopy of the kinesin-14 GCN4-Kar3Vik1 complexe... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5417 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-electron microscopy of the kinesin-14 GCN4-Kar3Vik1 complexed to microtubules in the AMP-PNP state (represents the ATP bound state) | |||||||||
 Map data Map data | Reconstruction of the kinesin-14 GCN4-Kar3Vik1 bound to microtubules in the AMP-PNP state, representative of the ATP-bound state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Kar3Vik1 / kinesin-14 / microtubule / spindle stabilization in mitosis | |||||||||
| Biological species |   | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 24.5 Å | |||||||||
 Authors Authors | Cope J / Rank KC / Gilbert S / Rayment I / Hoenger A | |||||||||
 Citation Citation |  Journal: J Cell Biol / Year: 2012 Journal: J Cell Biol / Year: 2012Title: Kar3Vik1, a member of the kinesin-14 superfamily, shows a novel kinesin microtubule binding pattern. Authors: Katherine C Rank / Chun Ju Chen / Julia Cope / Ken Porche / Andreas Hoenger / Susan P Gilbert / Ivan Rayment /  Abstract: Kinesin-14 motors generate microtubule minus-end-directed force used in mitosis and meiosis. These motors are dimeric and operate with a nonprocessive powerstroke mechanism, but the role of the ...Kinesin-14 motors generate microtubule minus-end-directed force used in mitosis and meiosis. These motors are dimeric and operate with a nonprocessive powerstroke mechanism, but the role of the second head in motility has been unclear. In Saccharomyces cerevisiae, the Kinesin-14 Kar3 forms a heterodimer with either Vik1 or Cik1. Vik1 contains a motor homology domain that retains microtubule binding properties but lacks a nucleotide binding site. In this case, both heads are implicated in motility. Here, we show through structural determination of a C-terminal heterodimeric Kar3Vik1, electron microscopy, equilibrium binding, and motility that at the start of the cycle, Kar3Vik1 binds to or occludes two αβ-tubulin subunits on adjacent protofilaments. The cycle begins as Vik1 collides with the microtubule followed by Kar3 microtubule association and ADP release, thereby destabilizing the Vik1-microtubule interaction and positioning the motor for the start of the powerstroke. The results indicate that head-head communication is mediated through the adjoining coiled coil. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5417.map.gz emd_5417.map.gz | 10.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5417-v30.xml emd-5417-v30.xml emd-5417.xml emd-5417.xml | 13.8 KB 13.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5417.tif emd_5417.tif | 407.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5417 http://ftp.pdbj.org/pub/emdb/structures/EMD-5417 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5417 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5417 | HTTPS FTP |
-Validation report
| Summary document |  emd_5417_validation.pdf.gz emd_5417_validation.pdf.gz | 78.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5417_full_validation.pdf.gz emd_5417_full_validation.pdf.gz | 77.8 KB | Display | |
| Data in XML |  emd_5417_validation.xml.gz emd_5417_validation.xml.gz | 494 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5417 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5417 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5417 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5417 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5417.map.gz / Format: CCP4 / Size: 10.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5417.map.gz / Format: CCP4 / Size: 10.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of the kinesin-14 GCN4-Kar3Vik1 bound to microtubules in the AMP-PNP state, representative of the ATP-bound state | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.8 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : GCN4-Kar3Vik1 bound to microtubules in the AMP-PNP state
| Entire | Name: GCN4-Kar3Vik1 bound to microtubules in the AMP-PNP state |
|---|---|
| Components |
|
-Supramolecule #1000: GCN4-Kar3Vik1 bound to microtubules in the AMP-PNP state
| Supramolecule | Name: GCN4-Kar3Vik1 bound to microtubules in the AMP-PNP state type: sample / ID: 1000 Details: GCN4-Kar3Vik1 was incubated with the non-hydrolyzable ATP analog AMP-PNP to trap the motor in the ATP-state conformation. Oligomeric state: One heterodimer of Kar3Vik1 binds to one heterodimer of alpha-beta tubulin Number unique components: 2 |
|---|
-Macromolecule #1: GCN4-Kar3Vik1
| Macromolecule | Name: GCN4-Kar3Vik1 / type: protein_or_peptide / ID: 1 Details: This truncated version of Kar3Vik1 contains the complete C-terminal globular domains as well as two and a half heptads of the native coiled coil. The GCN4 leucine zipper sequence was added ...Details: This truncated version of Kar3Vik1 contains the complete C-terminal globular domains as well as two and a half heptads of the native coiled coil. The GCN4 leucine zipper sequence was added to the N-terminus to initialize dimerization. Number of copies: 1 / Oligomeric state: Heterodimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 87 KDa / Theoretical: 87 KDa |
| Recombinant expression | Organism:  |
-Macromolecule #2: alpha-beta tubulin
| Macromolecule | Name: alpha-beta tubulin / type: protein_or_peptide / ID: 2 / Details: Heterodimer of alpha and beta tubulin / Number of copies: 1 / Oligomeric state: Heterodimer / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 110 KDa / Theoretical: 110 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 0.70 mg/mL |
|---|---|
| Buffer | pH: 7.2 Details: 20mM HEPES, 5mM magnesium acetate, 50mM potassium acetate, 0.1mM EDTA, 0.1mM EGTA, 1mM DTT |
| Grid | Details: C-flat 200 mesh copper grid with holey carbon film. |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 93 K / Instrument: HOMEMADE PLUNGER / Details: Vitrification carried out at room temperature Method: 5 uL of 0.41 mg/mL microtubules was adsorbed to a grid for 45 seconds. Excess liquid was blotted away and immediately 5 uL of 0.70 mg/mL Kar3Vik1 complexed with AMP-PNP was added to the ...Method: 5 uL of 0.41 mg/mL microtubules was adsorbed to a grid for 45 seconds. Excess liquid was blotted away and immediately 5 uL of 0.70 mg/mL Kar3Vik1 complexed with AMP-PNP was added to the microtubules for 2 minutes. Excess liquid was blotted for approximately 2.5 seconds prior to plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Temperature | Min: 95 K / Max: 97 K / Average: 96 K |
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 100,000 times magnification. |
| Details | Low-dose cryo-EM recording |
| Date | Jun 8, 2011 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Digitization - Sampling interval: 15 µm / Number real images: 34 / Average electron dose: 15 e/Å2 / Details: Recorded on CCD 4K camera / Od range: 1.4 / Bits/pixel: 14 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder: GATAN 626 cryo-holder / Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Helical processing was carried out with PHOELIX |
|---|---|
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 10.666 Å Applied symmetry - Helical parameters - Δ&Phi: 24 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 24.5 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: IMOD, PHOELIX, SUPRIM Details: Final map was calculated from an average of 67 datasets including approximately 27000 asymmetric units. |
 Movie
Movie Controller
Controller


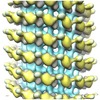





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















