+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5405 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Icosahedral reconstruction of bacteriophage P4 procapsid | |||||||||
 Map data Map data | Reconstruction of P4 procapsid | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | bacteriophage / P2 / P4 / capsid / procapsid / assembly / size determination / scaffolding protein / triangulation numbers / T=7 dextro / Sid / gpN | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 8.0 Å | |||||||||
 Authors Authors | Dearborn AD / Laurinmaki P / Chandramouli P / Rodenburg CM / Wang S / Butcher SJ / Dokland T | |||||||||
 Citation Citation |  Journal: J Struct Biol / Year: 2012 Journal: J Struct Biol / Year: 2012Title: Structure and size determination of bacteriophage P2 and P4 procapsids: function of size responsiveness mutations. Authors: Altaira D Dearborn / Pasi Laurinmaki / Preethi Chandramouli / Cynthia M Rodenburg / Sifang Wang / Sarah J Butcher / Terje Dokland /  Abstract: Bacteriophage P4 is dependent on structural proteins supplied by a helper phage, P2, to assemble infectious virions. Bacteriophage P2 normally forms an icosahedral capsid with T=7 symmetry from the ...Bacteriophage P4 is dependent on structural proteins supplied by a helper phage, P2, to assemble infectious virions. Bacteriophage P2 normally forms an icosahedral capsid with T=7 symmetry from the gpN capsid protein, the gpO scaffolding protein and the gpQ portal protein. In the presence of P4, however, the same structural proteins are assembled into a smaller capsid with T=4 symmetry. This size determination is effected by the P4-encoded protein Sid, which forms an external scaffold around the small P4 procapsids. Size responsiveness (sir) mutants in gpN fail to assemble small capsids even in the presence of Sid. We have produced large and small procapsids by co-expression of gpN with gpO and Sid, respectively, and applied cryo-electron microscopy and three-dimensional reconstruction methods to visualize these procapsids. gpN has an HK97-like fold and interacts with Sid in an exposed loop where the sir mutations are clustered. The T=7 lattice of P2 has dextro handedness, unlike the laevo lattices of other phages with this fold observed so far. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5405.map.gz emd_5405.map.gz | 22.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5405-v30.xml emd-5405-v30.xml emd-5405.xml emd-5405.xml | 11.4 KB 11.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5405_1.jpg emd_5405_1.jpg | 111.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5405 http://ftp.pdbj.org/pub/emdb/structures/EMD-5405 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5405 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5405 | HTTPS FTP |
-Validation report
| Summary document |  emd_5405_validation.pdf.gz emd_5405_validation.pdf.gz | 78.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5405_full_validation.pdf.gz emd_5405_full_validation.pdf.gz | 77.2 KB | Display | |
| Data in XML |  emd_5405_validation.xml.gz emd_5405_validation.xml.gz | 492 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5405 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5405 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5405 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5405 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5405.map.gz / Format: CCP4 / Size: 38.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5405.map.gz / Format: CCP4 / Size: 38.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of P4 procapsid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.73 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
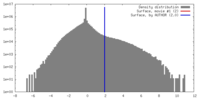
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Satellite bacteriophage P4 procapsid
| Entire | Name: Satellite bacteriophage P4 procapsid |
|---|---|
| Components |
|
-Supramolecule #1000: Satellite bacteriophage P4 procapsid
| Supramolecule | Name: Satellite bacteriophage P4 procapsid / type: sample / ID: 1000 / Oligomeric state: 240 copies of gpN and 60 copies of Sid / Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 11.268 MDa |
-Macromolecule #1: gpN
| Macromolecule | Name: gpN / type: protein_or_peptide / ID: 1 / Name.synonym: N*, major capsid protein / Oligomeric state: icosahedral / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 9.648 MDa |
| Recombinant expression | Organism:  |
-Macromolecule #2: Sid
| Macromolecule | Name: Sid / type: protein_or_peptide / ID: 2 / Name.synonym: external scaffolding protein / Oligomeric state: icosahedral / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 1.62 MDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 10 mM Tris-HCl, pH 8.0, 20 mM NaCl, 1 mM MgCl2 |
| Grid | Details: 200 mesh Quantifoil R2/2 |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 110 K / Instrument: HOMEMADE PLUNGER / Method: Blot for 2 sec before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Temperature | Min: 95 K / Max: 99 K / Average: 97 K |
| Date | Jan 1, 1999 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 4.545 µm / Average electron dose: 20 e/Å2 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 50000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Details: Each micrograph |
|---|---|
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 8.0 Å / Resolution method: OTHER / Software - Name: EMAN,AUTO3DEM / Number images used: 2286 |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: B |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | Protocol: Rigid body fitting of individual subsegments |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)