+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | (+)ML-SI3 bound TRPML2 in an open state | |||||||||
 Map data Map data | ML-SI3 bound TRPML2 in an open state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRPML / calcium channel / ion transport / membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of macrophage inflammatory protein 1 alpha production / macrophage migration / NAADP-sensitive calcium-release channel activity / positive regulation of chemokine (C-C motif) ligand 5 production / iron ion transmembrane transporter activity / neutrophil migration / positive regulation of monocyte chemotactic protein-1 production / positive regulation of chemokine (C-X-C motif) ligand 2 production / TRP channels / calcium ion transmembrane transport ...positive regulation of macrophage inflammatory protein 1 alpha production / macrophage migration / NAADP-sensitive calcium-release channel activity / positive regulation of chemokine (C-C motif) ligand 5 production / iron ion transmembrane transporter activity / neutrophil migration / positive regulation of monocyte chemotactic protein-1 production / positive regulation of chemokine (C-X-C motif) ligand 2 production / TRP channels / calcium ion transmembrane transport / calcium channel activity / recycling endosome membrane / late endosome membrane / protein transport / adaptive immune response / lysosome / innate immune response / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.61 Å | |||||||||
 Authors Authors | Schmiege P / Li X | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: TRPML2 in distinct states reveals the activation and modulation principles of the TRPML family. Authors: Philip Schmiege / Dawid Jaślan / Michael Fine / Nidish Ponath Sadanandan / Alexandra Hatton / Nadia Elghobashi-Meinhardt / Christian Grimm / Xiaochun Li /     Abstract: TRPML2 activity is critical for endolysosomal integrity and chemokine secretion, and can be modulated by various ligands. Interestingly, two ML-SI3 isomers regulate TRPML2 oppositely. The molecular ...TRPML2 activity is critical for endolysosomal integrity and chemokine secretion, and can be modulated by various ligands. Interestingly, two ML-SI3 isomers regulate TRPML2 oppositely. The molecular mechanism underlying this unique isomeric preference as well as the TRPML2 agonistic mechanism remains unknown. Here, we present six cryo-EM structures of human TRPML2 in distinct states revealing that the π-bulge of the S6 undergoes a π-α transition upon agonist binding, highlighting the remarkable role of the π-bulge in ion channel regulation. Moreover, we identify that PI(3,5)P allosterically affects the pose of ML2-SA1, a TRPML2 specific activator, resulting in an open channel without the π-α transition. Functional and structural studies show that mutating the S5 of TRPML1 to that of TRPML2 enables the mutated TRPML1 to be activated by (+)ML-SI3 and ML2-SA1. Thus, our work elucidates the activation mechanism of TRPML channels and paves the way for the development of selective TRPML modulators. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_48142.map.gz emd_48142.map.gz | 64.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-48142-v30.xml emd-48142-v30.xml emd-48142.xml emd-48142.xml | 18.3 KB 18.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_48142.png emd_48142.png | 47 KB | ||
| Filedesc metadata |  emd-48142.cif.gz emd-48142.cif.gz | 6.1 KB | ||
| Others |  emd_48142_half_map_1.map.gz emd_48142_half_map_1.map.gz emd_48142_half_map_2.map.gz emd_48142_half_map_2.map.gz | 115.5 MB 115.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-48142 http://ftp.pdbj.org/pub/emdb/structures/EMD-48142 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-48142 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-48142 | HTTPS FTP |
-Related structure data
| Related structure data |  9ekzMC  9eksC  9ektC  9ekuC  9ekvC  9ekwC  9ekxC  9ekyC  9el0C  9el1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_48142.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_48142.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ML-SI3 bound TRPML2 in an open state | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.936 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map A
| File | emd_48142_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map B
| File | emd_48142_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : (+)ML-SI3 bound TRPML2
| Entire | Name: (+)ML-SI3 bound TRPML2 |
|---|---|
| Components |
|
-Supramolecule #1: (+)ML-SI3 bound TRPML2
| Supramolecule | Name: (+)ML-SI3 bound TRPML2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Mucolipin-2
| Macromolecule | Name: Mucolipin-2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 66.016297 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MARQPYRFPQ ARIPERGSGV FRLTVRNAMA HRDSEMKEEC LREDLKFYFM SPCEKYRARR QIPWKLGLQI LKIVMVTTQL VRFGLSNQL VVAFKEDNTV AFKHLFLKGY SGTDEDDYSC SVYTQEDAYE SIFFAINQYH QLKDITLGTL GYGENEDNRI G LKVCKQHY ...String: MARQPYRFPQ ARIPERGSGV FRLTVRNAMA HRDSEMKEEC LREDLKFYFM SPCEKYRARR QIPWKLGLQI LKIVMVTTQL VRFGLSNQL VVAFKEDNTV AFKHLFLKGY SGTDEDDYSC SVYTQEDAYE SIFFAINQYH QLKDITLGTL GYGENEDNRI G LKVCKQHY KKGTMFPSNE TLNIDNDVEL DCVQLDLQDL SKKPPDWKNS SFFRLEFYRL LQVEISFHLK GIDLQTIHSR EL PDCYVFQ NTIIFDNKAH SGKIKIYFDS DAKIEECKDL NIFGSTQKNA QYVLVFDAFV IVICLASLIL CTRSIVLALR LRK RFLNFF LEKYKRPVCD TDQWEFINGW YVLVIISDLM TIIGSILKME IKAKNLTNYD LCSIFLGTST LLVWVGVIRY LGYF QAYNV LILTMQASLP KVLRFCACAG MIYLGYTFCG WIVLGPYHDK FENLNTVAEC LFSLVNGDDM FATFAQIQQK SILVW LFSR LYLYSFISLF IYMILSLFIA LITDSYDTIK KFQQNGFPET DLQEFLKECS SKEEYQKESS AFLSCICCRR RKRSDD HLI PIS UniProtKB: Mucolipin-2 |
-Macromolecule #2: N-{(1S,2S)-2-[4-(2-methoxyphenyl)piperazin-1-yl]cyclohexyl}benzen...
| Macromolecule | Name: N-{(1S,2S)-2-[4-(2-methoxyphenyl)piperazin-1-yl]cyclohexyl}benzenesulfonamide type: ligand / ID: 2 / Number of copies: 4 / Formula: ZB4 |
|---|---|
| Molecular weight | Theoretical: 429.576 Da |
| Chemical component information | 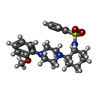 ChemComp-ZB4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































