[English] 日本語
 Yorodumi
Yorodumi- EMDB-4780: Heterodimeric ABC exporter TmrAB under turnover conditions in asy... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4780 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Heterodimeric ABC exporter TmrAB under turnover conditions in asymmetric unlocked return conformation with wider opened intracellular gate | ||||||||||||
 Map data Map data | TmrAB in asymmetric unlocked return state under turnover conditions with wider opening of intracellular gate | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | ATP-binding cassette transporter / membrane protein / heterodimer / exporter / TRANSPORT PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationABC-type oligopeptide transporter activity / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |   Thermus thermophilus (bacteria) / Thermus thermophilus (bacteria) /  | ||||||||||||
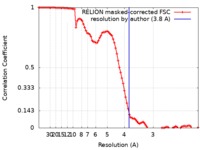
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | ||||||||||||
 Authors Authors | Januliene D / Hofmann S / Medhdipour AR / Thomas C / Hummer G / Tampe R / Moeller A | ||||||||||||
| Funding support |  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Conformation space of a heterodimeric ABC exporter under turnover conditions. Authors: Susanne Hofmann / Dovile Januliene / Ahmad R Mehdipour / Christoph Thomas / Erich Stefan / Stefan Brüchert / Benedikt T Kuhn / Eric R Geertsma / Gerhard Hummer / Robert Tampé / Arne Moeller /  Abstract: Cryo-electron microscopy (cryo-EM) has the capacity to capture molecular machines in action. ATP-binding cassette (ABC) exporters are highly dynamic membrane proteins that extrude a wide range of ...Cryo-electron microscopy (cryo-EM) has the capacity to capture molecular machines in action. ATP-binding cassette (ABC) exporters are highly dynamic membrane proteins that extrude a wide range of substances from the cytosol and thereby contribute to essential cellular processes, adaptive immunity and multidrug resistance. Despite their importance, the coupling of nucleotide binding, hydrolysis and release to the conformational dynamics of these proteins remains poorly resolved, especially for heterodimeric and/or asymmetric ABC exporters that are abundant in humans. Here we present eight high-resolution cryo-EM structures that delineate the full functional cycle of an asymmetric ABC exporter in a lipid environment. Cryo-EM analysis under active turnover conditions reveals distinct inward-facing (IF) conformations-one of them with a bound peptide substrate-and previously undescribed asymmetric post-hydrolysis states with dimerized nucleotide-binding domains and a closed extracellular gate. By decreasing the rate of ATP hydrolysis, we could capture an outward-facing (OF) open conformation-an otherwise transient state vulnerable to substrate re-entry. The ATP-bound pre-hydrolysis and vanadate-trapped states are conformationally equivalent; both comprise co-existing OF conformations with open and closed extracellular gates. By contrast, the post-hydrolysis states from the turnover experiment exhibit asymmetric ATP and ADP occlusion after phosphate release from the canonical site and display a progressive separation of the nucleotide-binding domains and unlocking of the intracellular gate. Our findings reveal that phosphate release, not ATP hydrolysis, triggers the return of the exporter to the IF conformation. By mapping the conformational landscape during active turnover, aided by mutational and chemical modulation of kinetic rates to trap the key intermediates, we resolved fundamental steps of the substrate translocation cycle of asymmetric ABC transporters. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4780.map.gz emd_4780.map.gz | 3.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4780-v30.xml emd-4780-v30.xml emd-4780.xml emd-4780.xml | 26.9 KB 26.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4780_fsc.xml emd_4780_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_4780.png emd_4780.png | 41.4 KB | ||
| Masks |  emd_4780_msk_1.map emd_4780_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-4780.cif.gz emd-4780.cif.gz | 7.9 KB | ||
| Others |  emd_4780_half_map_1.map.gz emd_4780_half_map_1.map.gz emd_4780_half_map_2.map.gz emd_4780_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4780 http://ftp.pdbj.org/pub/emdb/structures/EMD-4780 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4780 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4780 | HTTPS FTP |
-Related structure data
| Related structure data |  6ramMC  4773C  4774C  4775C  4776C  4777C  4778C  4779C  4781C  6rafC  6ragC  6rahC  6raiC  6rajC  6rakC  6ralC  6ranC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4780.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4780.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TmrAB in asymmetric unlocked return state under turnover conditions with wider opening of intracellular gate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.077 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_4780_msk_1.map emd_4780_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2
| File | emd_4780_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1
| File | emd_4780_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : TmrAB under turnover conditions in asymmetric unlocked return con...
| Entire | Name: TmrAB under turnover conditions in asymmetric unlocked return conformation with wider opened intracellular gate |
|---|---|
| Components |
|
-Supramolecule #1: TmrAB under turnover conditions in asymmetric unlocked return con...
| Supramolecule | Name: TmrAB under turnover conditions in asymmetric unlocked return conformation with wider opened intracellular gate type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Molecular weight | Theoretical: 150 KDa |
-Supramolecule #2: TmrAB
| Supramolecule | Name: TmrAB / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
-Supramolecule #3: nanobody
| Supramolecule | Name: nanobody / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Multidrug resistance ABC transporter ATP-binding and permease protein
| Macromolecule | Name: Multidrug resistance ABC transporter ATP-binding and permease protein type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
| Molecular weight | Theoretical: 70.664797 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTEDTYSKAF DRALFARILR YVWPYRLQVV LALLFLLVVT LAAAATPLFF KWAIDLALVP TEPRPLAERF HLLLWISLGF LAVRAVHFA ATYGETYLIQ WVGQRVLFDL RSDLFAKLMR LHPGFYDRNP VGRLMTRVTS DVDAINQFIT GGLVGVIADL F TLVGLLGF ...String: MTEDTYSKAF DRALFARILR YVWPYRLQVV LALLFLLVVT LAAAATPLFF KWAIDLALVP TEPRPLAERF HLLLWISLGF LAVRAVHFA ATYGETYLIQ WVGQRVLFDL RSDLFAKLMR LHPGFYDRNP VGRLMTRVTS DVDAINQFIT GGLVGVIADL F TLVGLLGF MLFLSPKLTL VVLLVAPVLL AVTTWVRLGM RSAYREMRLR LARVNAALQE NLSGVETIQL FVKEREREEK FD RLNRDLF RAWVEIIRWF ALFFPVVGFL GDFAVASLVY YGGGEVVRGA VSLGLLVAFV DYTRQLFQPL QDLSDKFNLF QGA MASAER IFGVLDTEEE LKDPEDPTPI RGFRGEVEFR DVWLAYTPKG VEPTEKDWVL KGVSFRVRPG EKVALVGATG AGKT SVVSL IARFYDPQRG CVFLDGVDVR RYRQEELRRH VGIVLQEPFL FSGTVLDNLR LFDPSVPPER VEEVARFLGA HEFIL RLPK GYQTVLGERG AGLSTGEKQL LALVRALLAS PDILLILDEA TASVDSETEK RLQEALYKAM EGRTSLIIAH RLSTIR HVD RILVFRKGRL VEEGSHEELL AKGGYYAALY RLQFQEAKLG GGGENLYFQG HHHHHHHHHH UniProtKB: Multidrug resistance ABC transporter ATP-binding and permease protein |
-Macromolecule #2: Multidrug resistance ABC transporter ATP-binding and permease protein
| Macromolecule | Name: Multidrug resistance ABC transporter ATP-binding and permease protein type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermus thermophilus (bacteria) Thermus thermophilus (bacteria) |
| Molecular weight | Theoretical: 64.634457 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTGRSAAPLL RRLWPYVGRY RWRYLWAVLA GLVSIFFFVL TPYFLRLAVD AVQAGRGFGV YALAIVASAA LSGLLSYAMR RLAVVASRQ VEYDLRRDLL HHLLTLDRDF YHKHRVGDLM NRLNTDLSAV REMVGPGILM GSRLSFLVLL AFLSMYAVNA R LAFYLTLI ...String: MTGRSAAPLL RRLWPYVGRY RWRYLWAVLA GLVSIFFFVL TPYFLRLAVD AVQAGRGFGV YALAIVASAA LSGLLSYAMR RLAVVASRQ VEYDLRRDLL HHLLTLDRDF YHKHRVGDLM NRLNTDLSAV REMVGPGILM GSRLSFLVLL AFLSMYAVNA R LAFYLTLI LPGIFLAMRF LLRLIDRRYR EAQEVFDRIS TLAQEAFSGI RVVKGYALER RMVAWFQDLN RLYVEKSLAL AR VEGPLHA LLGFLMGFAF LTVLWAGGAM VVRGELSVGE LVQFNAYLAQ LTWPILGLGW VMALYQRGLT SLRRLFELLD EKP AIRDED PLPLALEDLS GEVRFEGVGL KRDGRWLLRG LTLTIPEGMT LGITGRTGSG KSLLAALVPR LLDPSEGRVY VGGH EARRI PLAVLRKAVG VAPQEPFLFS ETILENIAFG LDEVDRERVE WAARLAGIHE EILAFPKGYE TVLGERGITL SGGQR QRVA LARALAKRPK ILILDDALSA VDAETEARIL QGLKTVLGKQ TTLLISHRTA ALRHADWIIV LDGGRIVEEG THESLL QAG GLYAEMDRLQ KEVEA UniProtKB: Multidrug resistance ABC transporter ATP-binding and permease protein |
-Macromolecule #3: Nanobody Nb9F10
| Macromolecule | Name: Nanobody Nb9F10 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 14.594405 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAQLQLVESG GGLVQPGDSL RLSCAVSGSA LDYNAIGWFR QAPGKEREGV ACISKITGNT AYADSVKGRF TISRDNAKNT VHLQMNSLK PEDTAVYYCA TVTAVLLPGR CVPGKYWGQG TPVTVSSHHH HHHEPEA |
-Macromolecule #4: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 1 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 1 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil, UltrAuFoil / Material: GOLD / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average exposure time: 8.0 sec. / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)