+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HsSTING with SR-717 and C53 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Innate immunity / membrane protein / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationSTING complex / STAT6-mediated induction of chemokines / protein localization to endoplasmic reticulum / 2',3'-cyclic GMP-AMP binding / cyclic-di-GMP binding / STING mediated induction of host immune responses / serine/threonine protein kinase complex / positive regulation of type I interferon-mediated signaling pathway / IRF3-mediated induction of type I IFN / cGAS/STING signaling pathway ...STING complex / STAT6-mediated induction of chemokines / protein localization to endoplasmic reticulum / 2',3'-cyclic GMP-AMP binding / cyclic-di-GMP binding / STING mediated induction of host immune responses / serine/threonine protein kinase complex / positive regulation of type I interferon-mediated signaling pathway / IRF3-mediated induction of type I IFN / cGAS/STING signaling pathway / proton channel activity / reticulophagy / pattern recognition receptor signaling pathway / cytoplasmic pattern recognition receptor signaling pathway / cellular response to exogenous dsRNA / protein complex oligomerization / autophagosome membrane / positive regulation of macroautophagy / autophagosome assembly / : / cellular response to interferon-beta / positive regulation of type I interferon production / positive regulation of defense response to virus by host / endoplasmic reticulum-Golgi intermediate compartment membrane / signaling adaptor activity / antiviral innate immune response / activation of innate immune response / positive regulation of interferon-beta production / autophagosome / protein serine/threonine kinase binding / Regulation of innate immune responses to cytosolic DNA / secretory granule membrane / cytoplasmic vesicle membrane / SARS-CoV-1 activates/modulates innate immune responses / peroxisome / regulation of inflammatory response / defense response to virus / DNA-binding transcription factor binding / RNA polymerase II-specific DNA-binding transcription factor binding / mitochondrial outer membrane / endosome / cilium / ciliary basal body / Golgi membrane / innate immune response / ubiquitin protein ligase binding / Neutrophil degranulation / protein kinase binding / endoplasmic reticulum membrane / perinuclear region of cytoplasm / SARS-CoV-2 activates/modulates innate and adaptive immune responses / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / nucleoplasm / identical protein binding / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.09 Å | |||||||||
 Authors Authors | Gharpure A / Sulpizio A / Lairson LL / Ward AB | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Distinct oligomeric assemblies of STING induced by non-nucleotide agonists. Authors: Anant Gharpure / Ariana Sulpizio / Johannes R Loeffler / Monica L Fernández-Quintero / Andy S Tran / Luke L Lairson / Andrew B Ward /  Abstract: STING plays essential roles coordinating innate immune responses to processes that range from pathogenic infection to genomic instability. Its adaptor function is activated by cyclic dinucleotide ...STING plays essential roles coordinating innate immune responses to processes that range from pathogenic infection to genomic instability. Its adaptor function is activated by cyclic dinucleotide (CDN) secondary messengers originating from self (2'3'-cGAMP) or bacterial sources (3'3'-CDNs). Different classes of CDNs possess distinct binding modes, stabilizing STING's ligand-binding domain (LBD) in either a closed or open conformation. The closed conformation, induced by the endogenous ligand 2'3'-cGAMP, has been extensively studied using cryo-EM. However, significant questions remain regarding the structural basis of STING activation by open conformation-inducing ligands. Using cryo-EM, we investigate potential differences in conformational changes and oligomeric assemblies of STING for closed and open conformation-inducing synthetic agonists. While we observe a characteristic 180° rotation for both classes, the open-LBD inducing agonist diABZI-3 uniquely induces a quaternary structure reminiscent but distinct from the reported autoinhibited state of apo-STING. Additionally, we observe slower rates of activation for this ligand class in functional assays, which collectively suggests the existence of a potential additional regulatory mechanism for open conformation-inducing ligands that involves head-to-head interactions and restriction of curved oligomer formation. These observations have potential implications in the selection of an optimal class of STING agonist in the context of a defined therapeutic application. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_45897.map.gz emd_45897.map.gz | 117.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-45897-v30.xml emd-45897-v30.xml emd-45897.xml emd-45897.xml | 18.4 KB 18.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_45897.png emd_45897.png | 76.4 KB | ||
| Filedesc metadata |  emd-45897.cif.gz emd-45897.cif.gz | 6.3 KB | ||
| Others |  emd_45897_half_map_1.map.gz emd_45897_half_map_1.map.gz emd_45897_half_map_2.map.gz emd_45897_half_map_2.map.gz | 115.8 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-45897 http://ftp.pdbj.org/pub/emdb/structures/EMD-45897 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-45897 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-45897 | HTTPS FTP |
-Related structure data
| Related structure data |  9ct3MC  9ct4C  9ct5C  9ct6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_45897.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_45897.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.718 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_45897_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_45897_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Stimulator of interferon genes
| Entire | Name: Stimulator of interferon genes |
|---|---|
| Components |
|
-Supramolecule #1: Stimulator of interferon genes
| Supramolecule | Name: Stimulator of interferon genes / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 400 KDa |
-Macromolecule #1: Stimulator of interferon genes protein
| Macromolecule | Name: Stimulator of interferon genes protein / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.246 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MPHSSLHPSI PCPRGHGAQK AALVLLSACL VTLWGLGEPP EHTLRYLVLH LASLQLGLLL NGVCSLAEEL RHIHSRYRGS YWRTVRACL GCPLRRGALL LLSIYFYYSL PNAVGPPFTW MLALLGLSQA LNILLGLKGL APAEISAVCE KGNFNVAHGL A WSYYIGYL ...String: MPHSSLHPSI PCPRGHGAQK AALVLLSACL VTLWGLGEPP EHTLRYLVLH LASLQLGLLL NGVCSLAEEL RHIHSRYRGS YWRTVRACL GCPLRRGALL LLSIYFYYSL PNAVGPPFTW MLALLGLSQA LNILLGLKGL APAEISAVCE KGNFNVAHGL A WSYYIGYL RLILPELQAR IRTYNQHYNN LLRGAVSQRL YILLPLDCGV PDNLSMADPN IRFLDKLPQQ TGDHAGIKDR VY SNSIYEL LENGQRAGTC VLEYATPLQT LFAMSQYSQA GFSREDRLEQ AKLFCRTLED ILADAPESQN NCRLIAYQEP ADD SSFSLS QEVLRHLRQE EKEEVTVGGG SGGGSGGSAW SHPQFEK UniProtKB: Stimulator of interferon genes protein |
-Macromolecule #2: 4,5-difluoro-2-{[6-(1H-imidazol-1-yl)pyridazine-3-carbonyl]amino}...
| Macromolecule | Name: 4,5-difluoro-2-{[6-(1H-imidazol-1-yl)pyridazine-3-carbonyl]amino}benzoic acid type: ligand / ID: 2 / Number of copies: 4 / Formula: V67 |
|---|---|
| Molecular weight | Theoretical: 345.26 Da |
| Chemical component information | 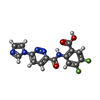 ChemComp-V67: |
-Macromolecule #3: 1-[(2-chloro-6-fluorophenyl)methyl]-3,3-dimethyl-2-oxo-N-[(2,4,6-...
| Macromolecule | Name: 1-[(2-chloro-6-fluorophenyl)methyl]-3,3-dimethyl-2-oxo-N-[(2,4,6-trifluorophenyl)methyl]-2,3-dihydro-1H-indole-6-carboxamide type: ligand / ID: 3 / Number of copies: 2 / Formula: 9IM |
|---|---|
| Molecular weight | Theoretical: 490.877 Da |
| Chemical component information | 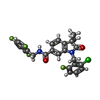 ChemComp-9IM: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: TFS FALCON 4i (4k x 4k) / Number real images: 4818 / Average exposure time: 2.2 sec. / Average electron dose: 45.07 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 20.0 µm / Illumination mode: SPOT SCAN / Imaging mode: OTHER / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 190000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)