[English] 日本語
 Yorodumi
Yorodumi- EMDB-45604: cryo-EM structure of calcineurin-fused beta2 adrenergic receptor ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryo-EM structure of calcineurin-fused beta2 adrenergic receptor in carazolol bound inactive state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / cryo-EM / calcineurin fusion / inactive state / MEMBRANE PROTEIN / MEMBRANE PROTEIN-HYDROLASE-ISOMERASE complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of angiotensin-activated signaling pathway / calcium-dependent protein serine/threonine phosphatase regulator activity / regulation of cell proliferation involved in kidney morphogenesis / CLEC7A (Dectin-1) induces NFAT activation / positive regulation of glomerulus development / negative regulation of calcium ion import across plasma membrane / negative regulation of signaling / calcium-dependent protein serine/threonine phosphatase activity / protein serine/threonine phosphatase complex / positive regulation of saliva secretion ...negative regulation of angiotensin-activated signaling pathway / calcium-dependent protein serine/threonine phosphatase regulator activity / regulation of cell proliferation involved in kidney morphogenesis / CLEC7A (Dectin-1) induces NFAT activation / positive regulation of glomerulus development / negative regulation of calcium ion import across plasma membrane / negative regulation of signaling / calcium-dependent protein serine/threonine phosphatase activity / protein serine/threonine phosphatase complex / positive regulation of saliva secretion / Calcineurin activates NFAT / calmodulin-dependent protein phosphatase activity / calcineurin complex / positive regulation of connective tissue replacement / positive regulation of calcium ion import across plasma membrane / Ca2+ pathway / FCERI mediated Ca+2 mobilization / negative regulation of dendrite morphogenesis / positive regulation of cardiac muscle hypertrophy in response to stress / macrolide binding / activin receptor binding / renal filtration / lung epithelial cell differentiation / calcineurin-NFAT signaling cascade / regulation of skeletal muscle contraction by regulation of release of sequestered calcium ion / cytoplasmic side of membrane / transforming growth factor beta receptor binding / TGFBR1 LBD Mutants in Cancer / positive regulation of calcineurin-NFAT signaling cascade / type I transforming growth factor beta receptor binding / signaling receptor inhibitor activity / transition between fast and slow fiber / negative regulation of activin receptor signaling pathway / myelination in peripheral nervous system / heart trabecula formation / positive regulation of osteoclast differentiation / I-SMAD binding / positive regulation of mini excitatory postsynaptic potential / cardiac muscle hypertrophy in response to stress / beta2-adrenergic receptor activity / regulation of synaptic vesicle cycle / negative regulation of smooth muscle contraction / AMPA selective glutamate receptor signaling pathway / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / norepinephrine binding / regulation of amyloid precursor protein catabolic process / Adrenoceptors / terminal cisterna / ryanodine receptor complex / positive regulation of lipophagy / negative regulation of G protein-coupled receptor signaling pathway / negative regulation of multicellular organism growth / 'de novo' protein folding / branching involved in blood vessel morphogenesis / response to psychosocial stress / adrenergic receptor signaling pathway / endosome to lysosome transport / ventricular cardiac muscle tissue morphogenesis / dendrite morphogenesis / protein dephosphorylation / protein-serine/threonine phosphatase / CLEC7A (Dectin-1) induces NFAT activation / regulation of postsynaptic neurotransmitter receptor internalization / diet induced thermogenesis / FK506 binding / positive regulation of cardiac muscle hypertrophy / parallel fiber to Purkinje cell synapse / positive regulation of cAMP/PKA signal transduction / TGF-beta receptor signaling activates SMADs / adenylate cyclase binding / regulation of ryanodine-sensitive calcium-release channel activity / positive regulation of activated T cell proliferation / mTORC1-mediated signalling / smooth muscle contraction / epithelial to mesenchymal transition / Calcineurin activates NFAT / positive regulation of endocytosis / epidermis development / Activation of BAD and translocation to mitochondria / DARPP-32 events / regulation of immune response / bone resorption / potassium channel regulator activity / positive regulation of bone mineralization / positive regulation of osteoblast differentiation / neuronal dense core vesicle / multicellular organismal response to stress / phosphatase binding / heart morphogenesis / postsynaptic modulation of chemical synaptic transmission / keratinocyte differentiation / intercellular bridge / skeletal muscle fiber development / regulation of sodium ion transport / adenylate cyclase-activating adrenergic receptor signaling pathway / supramolecular fiber organization / phosphoprotein phosphatase activity / brown fat cell differentiation Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Xu J / Chen G / Du Y / Kobilka BK | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: Calcineurin-fusion facilitates cryo-EM structure determination of a Family A GPCR. Authors: Jun Xu / Geng Chen / Haoqing Wang / Sheng Cao / Jie Heng / Xavier Deupi / Yang Du / Brian K Kobilka /    Abstract: Advances in singe-particle cryo-electron microscopy (cryo-EM) have made it possible to solve the structures of numerous Family A and Family B G protein-coupled receptors (GPCRs) in complex with G ...Advances in singe-particle cryo-electron microscopy (cryo-EM) have made it possible to solve the structures of numerous Family A and Family B G protein-coupled receptors (GPCRs) in complex with G proteins and arrestins, as well as several Family C GPCRs. Determination of these structures has been facilitated by the presence of large extramembrane components (such as G protein, arrestin, or Venus flytrap domains) in these complexes that aid in particle alignment during the processing of the cryo-EM data. In contrast, determination of the inactive state structure of Family A GPCRs is more challenging due to the relatively small size of the seven transmembrane domain (7TM) and to the surrounding detergent micelle that, in the absence of other features, make particle alignment impossible. Here, we describe an alternative protein engineering strategy where the heterodimeric protein calcineurin is fused to a GPCR by three points of attachment, the cytoplasmic ends of TM5, TM6, and TM7. This three-point attachment provides a more rigid link with the GPCR transmembrane domain that facilitates particle alignment during data processing, allowing us to determine the structures of the β adrenergic receptor (βAR) in the apo, antagonist-bound, and agonist-bound states. We expect that this fusion strategy may have broad application in cryo-EM structural determination of other Family A GPCRs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_45604.map.gz emd_45604.map.gz | 117.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-45604-v30.xml emd-45604-v30.xml emd-45604.xml emd-45604.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_45604.png emd_45604.png | 130.1 KB | ||
| Filedesc metadata |  emd-45604.cif.gz emd-45604.cif.gz | 6.5 KB | ||
| Others |  emd_45604_half_map_1.map.gz emd_45604_half_map_1.map.gz emd_45604_half_map_2.map.gz emd_45604_half_map_2.map.gz | 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-45604 http://ftp.pdbj.org/pub/emdb/structures/EMD-45604 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-45604 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-45604 | HTTPS FTP |
-Related structure data
| Related structure data |  9chxMC  9chuC  9chvC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_45604.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_45604.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_45604_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_45604_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : calcineurin fused beta2AR in complex with FKBP12
| Entire | Name: calcineurin fused beta2AR in complex with FKBP12 |
|---|---|
| Components |
|
-Supramolecule #1: calcineurin fused beta2AR in complex with FKBP12
| Supramolecule | Name: calcineurin fused beta2AR in complex with FKBP12 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: calcineurin fused beta2AR
| Supramolecule | Name: calcineurin fused beta2AR / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Protein phosphatase 3 catalytic subunit alpha
| Supramolecule | Name: Protein phosphatase 3 catalytic subunit alpha / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #4: FKBP1A
| Supramolecule | Name: FKBP1A / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Beta-2 adrenergic receptor,Calcineurin subunit B type 1
| Macromolecule | Name: Beta-2 adrenergic receptor,Calcineurin subunit B type 1 type: protein_or_peptide / ID: 1 Details: residues 29-354 (Uniprot numbering) of the beta2-AR with the third cytoplasmic domain replaced with residues 16-170 (Uniprot numbering) of calcineurin subunit B Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 51.309711 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DEVWVVGMGI VMSLIVLAIV FGNVLVITAI AKFERLQTVT NYFITSLACA DLVMGLAVVP FGAAHILMKM WTFGNFWCEF WTSIDVLCV TASIETLCVI AVDRYFAITS PFKYQSLLTK NKARVIILMV WIVSGLTSFL PIQMHWYRAT HQEAINCYAE E TCCDFFTN ...String: DEVWVVGMGI VMSLIVLAIV FGNVLVITAI AKFERLQTVT NYFITSLACA DLVMGLAVVP FGAAHILMKM WTFGNFWCEF WTSIDVLCV TASIETLCVI AVDRYFAITS PFKYQSLLTK NKARVIILMV WIVSGLTSFL PIQMHWYRAT HQEAINCYAE E TCCDFFTN QAYAIASSIV SFYVPLVIMV FVYSRVFQEA KRQLDADEIK RLGKRFKKLD LDNSGSLSVE EFMSLPELQQ NP LVQRVID IFDTDGNGEV DFKEFIEGVS QFSVKGDKEQ KLRFAFRIYD MDKDGYISNG ELFQVLKMMV GNNLKDTQLQ QIV DKTIIN ADKDGDGRIS FEEFCAVVGG LDIHKKMVVD VKFCLKEHKA LKTLGIIMGT FTLCWLPFFI VNIVHVIQDN LIRK EVYIL LNWIGYVNSG FNPLIYCRSP DFRIAFQELL CLRRDDLKAY GNGY UniProtKB: Beta-2 adrenergic receptor, Calcineurin subunit B type 1, Beta-2 adrenergic receptor |
-Macromolecule #2: Protein phosphatase 3 catalytic subunit alpha
| Macromolecule | Name: Protein phosphatase 3 catalytic subunit alpha / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: protein-serine/threonine phosphatase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42.627785 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SEPKAIDPKL STTDRVVKAV PFPPSHRLTA KEVFDNDGKP RVDILKAHLM KEGRLEESVA LRIITEGASI LRQEKNLLDI DAPVTVCGD IHGQFFDLMK LFEVGGSPAN TRYLFLGDYV DRGYFSIECV LYLWALKILY PKTLFLLRGN HECRHLTEYF T FKQECKIK ...String: SEPKAIDPKL STTDRVVKAV PFPPSHRLTA KEVFDNDGKP RVDILKAHLM KEGRLEESVA LRIITEGASI LRQEKNLLDI DAPVTVCGD IHGQFFDLMK LFEVGGSPAN TRYLFLGDYV DRGYFSIECV LYLWALKILY PKTLFLLRGN HECRHLTEYF T FKQECKIK YSERVYDACM DAFDCLPLAA LMNQQFLCVH GGLSPEINTL DDIRKLDRFK EPPAYGPMCD ILWSDPLEDF GN EKTQEHF THNTVRGCSY FYSYPAVCDF LQHNNLLSIL RAHEAQDAGY RMYRKSQTTG FPSLITIFSA PNYLDVYNNK AAV LKYENN VMNIRQFNCS PHPYWLPNFM DVFTWSLPFV GEKVTEMLVN VLNI UniProtKB: Protein phosphatase 3 catalytic subunit alpha |
-Macromolecule #3: Peptidyl-prolyl cis-trans isomerase FKBP1A
| Macromolecule | Name: Peptidyl-prolyl cis-trans isomerase FKBP1A / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: peptidylprolyl isomerase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.967705 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGVQVETISP GDGRTFPKRG QTCVVHYTGM LEDGKKFDSS RDRNKPFKFM LGKQEVIRGW EEGVAQMSVG QRAKLTISPD YAYGATGHP GIIPPHATLV FDVELLKLE UniProtKB: Peptidyl-prolyl cis-trans isomerase FKBP1A |
-Macromolecule #4: (2S)-1-(9H-Carbazol-4-yloxy)-3-(isopropylamino)propan-2-ol
| Macromolecule | Name: (2S)-1-(9H-Carbazol-4-yloxy)-3-(isopropylamino)propan-2-ol type: ligand / ID: 4 / Number of copies: 1 / Formula: CAU |
|---|---|
| Molecular weight | Theoretical: 298.379 Da |
| Chemical component information | 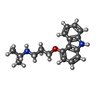 ChemComp-CAU: |
-Macromolecule #5: 8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN
| Macromolecule | Name: 8-DEETHYL-8-[BUT-3-ENYL]-ASCOMYCIN / type: ligand / ID: 5 / Number of copies: 1 / Formula: FK5 |
|---|---|
| Molecular weight | Theoretical: 804.018 Da |
| Chemical component information | 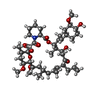 ChemComp-FK5: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 276093 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)