[English] 日本語
 Yorodumi
Yorodumi- EMDB-4541: Nanodisc reconstituted human ABCB1 in complex with UIC2 fab and taxol -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4541 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Nanodisc reconstituted human ABCB1 in complex with UIC2 fab and taxol | |||||||||
 Map data Map data | postprocessed map of human ABCB1 in complex with UIC2 fab and taxol after partial nanodisc signal subtraction. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
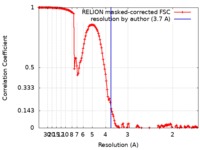
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Alam A / Locher KP | |||||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: Structural insight into substrate and inhibitor discrimination by human P-glycoprotein. Authors: Amer Alam / Julia Kowal / Eugenia Broude / Igor Roninson / Kaspar P Locher /   Abstract: ABCB1, also known as P-glycoprotein, actively extrudes xenobiotic compounds across the plasma membrane of diverse cells, which contributes to cellular drug resistance and interferes with therapeutic ...ABCB1, also known as P-glycoprotein, actively extrudes xenobiotic compounds across the plasma membrane of diverse cells, which contributes to cellular drug resistance and interferes with therapeutic drug delivery. We determined the 3.5-angstrom cryo-electron microscopy structure of substrate-bound human ABCB1 reconstituted in lipidic nanodiscs, revealing a single molecule of the chemotherapeutic compound paclitaxel (Taxol) bound in a central, occluded pocket. A second structure of inhibited, human-mouse chimeric ABCB1 revealed two molecules of zosuquidar occupying the same drug-binding pocket. Minor structural differences between substrate- and inhibitor-bound ABCB1 sites are amplified toward the nucleotide-binding domains (NBDs), revealing how the plasticity of the drug-binding site controls the dynamics of the adenosine triphosphate-hydrolyzing NBDs. Ordered cholesterol and phospholipid molecules suggest how the membrane modulates the conformational changes associated with drug binding and transport. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4541.map.gz emd_4541.map.gz | 224.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4541-v30.xml emd-4541-v30.xml emd-4541.xml emd-4541.xml | 10.6 KB 10.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4541_fsc.xml emd_4541_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_4541.png emd_4541.png | 140.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4541 http://ftp.pdbj.org/pub/emdb/structures/EMD-4541 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4541 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4541 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_4541.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4541.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | postprocessed map of human ABCB1 in complex with UIC2 fab and taxol after partial nanodisc signal subtraction. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Nanodisc reconstituted ABCB1HM (human mouse chimeric ABCB1) EQ mu...
| Entire | Name: Nanodisc reconstituted ABCB1HM (human mouse chimeric ABCB1) EQ mutant in complex with UIC2 Fab and zosuquidar |
|---|---|
| Components |
|
-Supramolecule #1: Nanodisc reconstituted ABCB1HM (human mouse chimeric ABCB1) EQ mu...
| Supramolecule | Name: Nanodisc reconstituted ABCB1HM (human mouse chimeric ABCB1) EQ mutant in complex with UIC2 Fab and zosuquidar type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Molecular weight | Experimental: 200 KDa |
-Supramolecule #2: nanodisc reconstituted human ABCB1 in complex with UIC2 Fab and taxol
| Supramolecule | Name: nanodisc reconstituted human ABCB1 in complex with UIC2 Fab and taxol type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: UIC2 Fab
| Supramolecule | Name: UIC2 Fab / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 2.1 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)